Endometrial cancer (EC) is the sixth most commonly occurring cancer in women and the 15th most common cancer overall [1]. There were over 417,000 new cases and 97,000 deaths in 2020 due to this cancer [2]. Several studies have shown that the risk of EC increases with older age, early menstruation, late menopause, diabetes mellitus, obesity, family history of EC, exposure to radiation, infertility (particularly linked to polycystic ovarian syndrome) and prolonged use of estrogen in hormone therapy [3–5]. The precise biological mechanisms linking diabetes to an increased risk of developing EC have not yet been completely understood. The underlying mechanisms, which may involve hyperglycemia, hyperinsulinemia, chronic inflammation, and the activation of specific signaling pathways, could participate in this process [6] (Supplementary Table SI). Earlier meta-analyses of clinical evidence support the association between metabolic syndrome, diabetes mellitus, and increased risk of EC [7–9], but to improve understanding we designed an updated systematic review and meta-analysis based on the latest studies so as to properly represent the scope of the association between diabetes and EC risk.
Material and methods
Study design and search strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [10]. The study was registered with the PROSPERO (ID: CRD42024568221). The Embase, Medline and the Cochrane Library databases were searched to identify case-control studies of diabetes mellitus and EC published between January 2000 and October 2023. Articles were selected using the following sets of keywords: “diabetes” OR “diabetes mellitus”; AND “endometrial cancer” OR “endometrial carcinoma”.
Selection criteria
Studies that met the following criteria were included in meta-analysis: case-control study design; full-text articles published between 01/01/2000 and 30/10/2023; included data on the relationship between diabetes and EC; incorporated raw data, i.e. data on the number of patients in each group, not just risk rates; data were sufficient to calculate the odds ratio (OR) and 95% confidence interval (CI); written in English.
Data extraction and quality assessment
Studies were excluded based on the following criteria: inappropriate design; were reviews, letters, case reports, commentaries; data for analysis were not available; the risk was related to metformin use; the articles did not show raw results; published in languages other than English.
Authors used the standardized data extraction form. The extracted information included: study design, first author, year of publication, number of cases and controls, study period, women’s age, type of diabetes (if available), and source of subjects. Two researchers (A.D. and W.K.) independently examined the titles and abstracts in accordance with the established inclusion criteria to exclude ineligible studies with conflicts resolved through discussion with the third author (M.M.).
The Newcastle Ottawa Scale (NOS) was applied to assess the quality of the included studies [11]. In the methodological evaluation process, scores from 0 to 3, from 4 to 6, and from 7 to 9 were given for low, medium and high quality, respectively. NOS includes 3 categories: selection, comparability and exposure. In scoring, each numbered item in the selection and exposure categories could receive up to 1 star, while comparability could receive a maximum of 2 stars [11].
Statistical analysis
The Statistica 13.3 software (StatSoft, Krakow, Poland) was used for all statistical analyses. We summarized the risk estimate from each study by employing a random-effects model with ORs. For each study, we created separate two-by-two crosstab containing the following data: number of patients with diabetes who developed EC/number of patients with diabetes who did not develop EC/number of patients without diabetes who developed EC/number of patients without diabetes who did not develop EC. Based on this, we calculated the OR and 95% CIs. The DerSimonian–Laird random-effects model was implemented [12]. Heterogeneity was assessed by employing a forest plot, and, statistically, by adopting the Q test, I2 index and p-value. The I2 statistic (> 75.0%, 50.0–75.0% and < 50% indicating substantial, moderate and low heterogeneity, respectively) was applied [13]. Publication bias was assessed by funnel plot, and estimated using Begg’s test and Egger’s test [14].
Additionally, a stratified subgroup analysis was performed to investigate which additional factors may influence the development of EC. The following variables were selected for analysis: family history of cancers, obesity, hypertension, age at menarche, menopausal status, parity, hormone replacement therapy, and oral contraceptive use.
Results
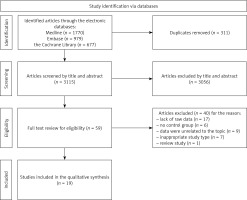
We identified 3,426 references through the medical databases. In accordance with inclusion criteria 3,056 articles were excluded by title, abstract, and 311 duplicates were removed. A total of 59 articles were identified for full-text review. Subsequently, comprehensive evaluation of publications resulted in the exclusion of 40 articles. Finally, we included 19 studies in the meta-analysis [15–33]. The study selection process is visually depicted in Figure 1.
Figure 1
Flow diagram of studies exploring the association between diabetes and EC risk presented in accordance with the PRISMA
Overall, 19 studies with a total of 57,824 participants (12,962 EC cases and 44,862 controls) were considered appropriate. Main characteristics of the studies are presented in Table I. Seven studies were conducted in the USA [17, 20, 25, 27–30], five in European countries [15, 22, 23, 26, 33], three in China [18, 24, 31], one study was conducted in Australia [19], one in Mexico [32], one in Canada [21] and one was a multicenter study [16]. The average NOS score was 6.63 for included studies (Supplementary Table SII).
Table I
Characteristics of the included studies
| First author [references] publication year | Location | Study period [years] | No. of cases/controls | Age (mean ± SD or range) | Source of subjects | NOS score |
|---|---|---|---|---|---|---|
| Esposito [15] 2021 | Italy | 1992–2006 | 454/908 | 19–79 | HBR | 8 |
| Dossus [16] 2021 | A number of countriesa | 2008–2012 | 853/853 | 63 ±7.9 | PB | 7 |
| Rodriguez [17] 2021 | Texas, USA | 2012–2016 | 201/19,664 | 45–70+ | PB | 8 |
| Gao [18] 2016 | Sanghai, China | 1997–2003 | 1,199/1,212 | 30–69 | PB | 6 |
| Nagle [19] 2013 | Australia | 2005–2007 | 1,398/1,538 | 18–79 | PB | 6 |
| Torres [20] 2012 | Minnesota, USA | 1985–2008 | 90/172 | 40–85 | HBR | 6 |
| Fiedenreich [21] 2011 | Canada | 2002–2006 | 515/962 | 30–79 | PB | 7 |
| Rosato [22] 2011 | Italy | 1992–2006 | 454/798 | 18–79 | HBR | 6 |
| Burbos [23] 2010 | United Kingdom | 2006–2009 | 149/2,898 | 59–72 | PBR | 5 |
| Zhang [24] 2010 | China | 2004–2008 | 942/1,721 | 40–70 | HBR | 8 |
| Fortuny [25] 2007 | USA | 2001–2005 | 469/467 | 33–88 | PB | 6 |
| Lucenteforte [26] 2007 | Italy, Switzerland | 1988–2006 | 777/1,550 | < 40–70+ | PBR | 8 |
| Saltzman [27] 2007 | USA | 1985–1999 | 1,303/1,779 | 45–74 | PBR | 7 |
| Strom [28] 2006 | USA | 1999–2002 | 511/1,412 | 50–79 | PB | 7 |
| Trentham-Dietz [29] 2006 | USA | 1992–1995 | 740/2,342 | 40–79 | PBR | 4 |
| Weiss [30] 2006 | USA | 1995–1999 | 1,281/1,704 | 45–74 | PBR | 5 |
| Xu [31] 2005 | China | 1997–2001 | 832/846 | 55.3±8.6 | PBR | 5 |
| Salazar-Martinez [32] 2000 | Mexico | 1995–1997 | 85/668 | < 40–71+ | HBR | 5 |
| Weiderpass [33] 2000 | Sweden | 1994–1995 | 709/3,368 | 50–74 | PB | 6 |
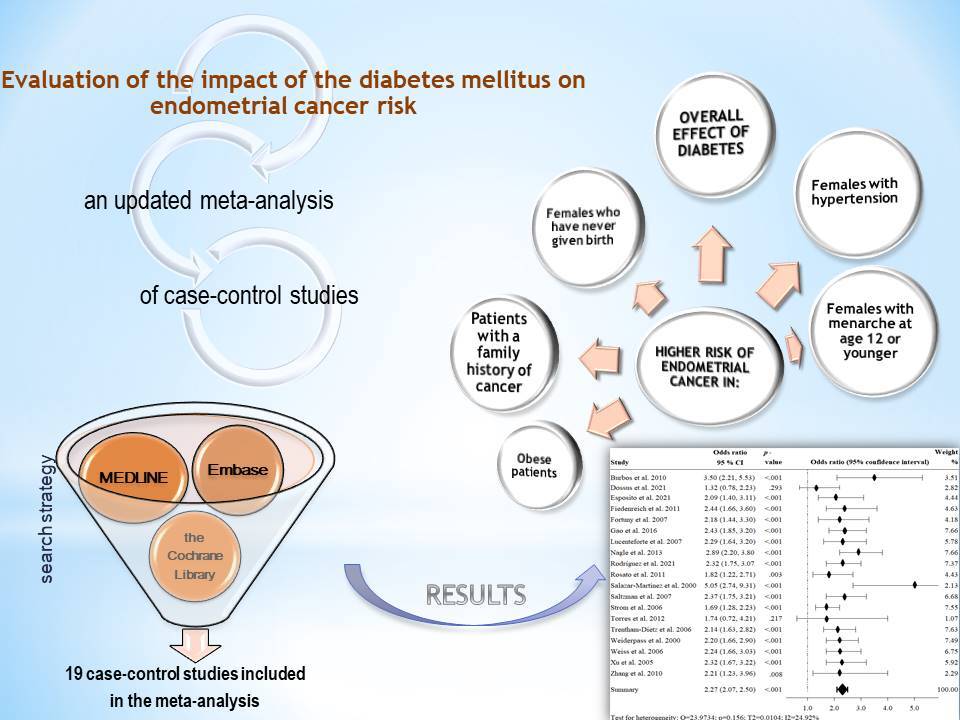
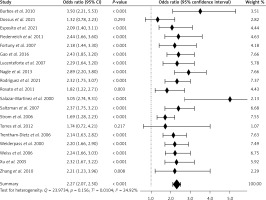
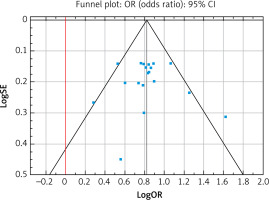
Figure 2 shows the risk of EC in people with diabetes compared to people without diabetes for selected studies. It turned out that the ORs of developing EC were 2.27 times higher (95% CI: 2.07–2.50) in patients with diabetes, and the results of the meta-analysis were statistically significant (p < 0.001). The I2 value was 24.92%, which means that the heterogeneity of the results was not significant (p < 0.157), and the Begg and Mazumdar’s rank correlation test (tau b = 0.064, Z = 0.385, p = 0.700) and Egger’s test (b0 = –0.242, 95% CI: 2.375–1.891, t = –0.240, p = 0.814) did not show publication bias. No obvious evidence of publication bias was detected by inspection of the funnel plot (Figure 3).
In order to deepen the analysis, a stratified subgroup analysis was performed. The ORs of developing EC were significantly higher in patients with a family history of cancer, obese patients, females with hypertension, females with menarche at the age of 12 or younger, and females who have never given birth. The study showed a significantly lower risk among females using oral contraceptives and among postmenopausal woman, while non-significant differences were seen between women who used hormone replacement therapy (Table II). It should be noted that the subgroup analysis should be approached with caution due to the high heterogeneity for the most covariates.
Table II
Modifying effects of other known factors on EC risk
Discussion
The conducted meta-analysis revealed that the risk of EC in women with diabetes is 2.27 times higher than in women without diabetes. Similar conclusions were drawn from meta-analyses conducted by Friberg et al. [8], (RR = 2.10, 95% CI: 1.75–2.53), and by Saed et al. [7], (RR = 1.85, 95% CI: 1.53–2.23). The heterogeneity of our meta-analysis (I2 = 24.92%) was, however, significantly lower than the study by Saed et al. (I2 = 66.7%) [7] and Friberg et al. (I2 = 48.8%) [8].
Elevated levels of insulin are frequently observed in individuals with diabetes, obesity and a lack of physical activity. Research has demonstrated that insulin, through its interaction with insulin receptors on endometrial cells, can promote the proliferation of endometrial stromal cells [34]. Higher levels of estradiol and estrogen are observed in women who are obese compared to those with a normal weight. This may contribute to the elevated risk of EC associated with obesity [35]. Obesity and a sedentary lifestyle are common risk factors for both type 2 diabetes and EC [36]. The conducted analysis demonstrated a risk of over three times higher for the development of EC in obese women (95% CI: 2.69–3.82, p < 0.001), similar conclusions were reached by Saed et al. (95% CI: 1.14–5.26, p < 0.022) [7]. Therefore, the higher risk of EC seen in individuals with diabetes may be influenced, at least in part, by the presence of these shared factors. Obesity causes accumulation of macrophages and heightened expression of pro-inflammatory cytokines [37]. An increase in pro-inflammatory cytokines contributes to insulin resistance and enhanced levels of insulin-like growth factor I. Obesity-induced systemic inflammation, dysregulated hormonal signaling and aberrations in insulin signaling, therefore, can increase the EC risk [38].
Meta-analyses of prospective observational studies have shown that a 5 kg/m2 increase in BMI is associated with a 60% increase in the relative risk of developing EC. This is particularly evident in an increasingly elderly population [39]. For postmenopausal women, adipose tissue becomes the dominant source of estrogen and, hence, plays a significant role in postmenopausal estrogen production [35]. Increased estrogen levels are not seen in premenopausal women who develop EC; instead a relative deficiency of progesterone appears to be important.
Studies conducted by numerous research groups have not shown a correlation between the use of antidiabetic medications and the risk of developing EC [40]. Metformin is a biguanide drug and the most commonly used oral hypoglycemic agent in type 2 diabetes mellitus [41]. The protective effect of metformin was consistent in various models, with a dose–response relationship showing a higher risk of endometrial cancer in diabetes patients versus non-diabetes people [42]. It has also been linked to the reversal of endometrial hyperplasia and may therefore contribute to decreasing the prevalence of endometrial carcinoma without the fertility and side effect consequences of current therapies [43]. Meta-analysis evaluated the effects of metformin in atypical endometrial hyperplasia, and, accordingly, EC patients show that metformin was associated with reversion of atypical endometrial hyperplasia to a normal endometrial, and with decreased cell proliferation biomarkers staining, from 51.94% (CI = 36.23% to 67.46%) to 34.47% (CI = 18.55% to 52.43%) [42]. For gynecological cancer patients, the beneficial effect of metformin use remains controversial due to inconsistent results being demonstrated in published articles [44, 45].
Limitations of the study should be pointed out. Firstly, we analyzed publications only in English which may introduce a language bias. Secondly, the lack of data regarding the types of diabetes should be noted. Only 6 of the included studies described the type of diabetes. Moreover, particular attention should be paid to metformin use. Additionally, it would be valuable to differentiate between precisely defined subcategories of EC. Another limitation may be found in discriminating between the mechanisms behind the relationship between diabetes and EC, the confounding impact of obesity, closely related to diabetes. It is worth mentioning that the studies did not include data such as physical activity, diet and socioeconomic factors. Finally, limitations were posed by publication high statistical heterogeneity in subgroup analyses.
In conclusion, meta-analysis of the currently available clinical evidence supports the association between diabetes mellitus and increased risk of EC. In addition, other coexisting modifiable factors may increase or decrease the risk of EC, leading to worsening or improving women outcomes for this disease. Diabetes requires cancer screening, which may contribute to improving public health. Solidified/Established/In-depth understanding of the relationship between diabetes mellitus and the risk of EC is important for clarifying this cancer etiology, and the higher risk of EC should be taken into account when caring for patients with diabetes and during the implementation of screening programs.