Introduction
Like most European countries, Poland is located in a naturally iodine-deficient area [1]. Based on research conducted in the 1980s, this region was classified as an area of mild-to-moderate iodine deficiency [2]. These data were the starting point for introducing actions to ensure proper iodine supply [3].
In 1997, the model of iodine prophylaxis based on consumption of iodized salt, which should contain 30 ±10 mg KI/kg of NaCl [4] or, since 2002, 39 ±13 mg KIO3/kg of NaCl, was introduced in Poland [5]. This model is still being continued. Although KIO3 is a more persistent compound, some experimental studies suggest that KI may provide more effective iodine prophylaxis than KIO3 [6].
Since then, the political and economic situation of Poland has also changed. Poland became a member of the European Union (in 2004), which was synonymous with the opening of the borders (common market) to the flow of goods, capital, services and people (Schengen area – since 2007). Political and economic changes have been followed by changes in the way of life. The population began to care about a “healthy” lifestyle, which resulted, among other things, in limiting dietary salt intake.
The aim of the study was to evaluate the effectiveness of iodine prophylaxis in Poland, based on over 20 years of observations of iodine supply, in a population of school-aged children in Opoczno.
Material and methods
The survey was conducted among children from a primary school in Opoczno, a town with a population of 23 thousand inhabitants, located in Central Poland, about 80 km southeast of Lodz. The study covered children from the entire Opoczno District attending the above-mentioned primary school.
A group of 603 randomly selected children (316 girls and 287 boys), Caucasian, born and growing up in Poland, aged 6–14, was examined. The study was conducted at 4 time points: in 1994, which was about 2.5 years before the introduction of iodine prophylaxis (88 children – 46 girls and 42 boys); in 1999 – 2.5 years after the introduction of iodine prophylaxis (207 children – 104 girls and 103 boys); in 2010 (170 children – 90 girls and 80 boys); and in 2016 (138 children – 76 girls and 62 boys); the two last time points were after Poland joined the European Union, and 13 and 19 years after the introduction of iodine prophylaxis, respectively.
The characteristics of the examined population are presented in Table I.
Table I
The number of examined children in gender, age and body surface area (BSA) groups
| Data | All | Age, mean ± SD [years] | BSA, mean ± SD [× 10–6 m] | Goitre incidence (age-adjusted) [%]a | Goitre incidence (BSA-adjusted) [%]b | Goitre incidence (age-adjusted) [%]c | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | ||
| 1994 | 88 | 9.74 ±1.66 | 1.14 ±0.20 | 92.6 | 95.4 | 66.7 | |||||
| 42 | 46 | 1.14 ±0.16 | 1.13 ±0.23 | 95.1 | 90 | 95.2 | 95.6 | 59 | 75 | ||
| 1999 | 207 | 9.69 ±2.35 | 1.17 ± 0.26 | 18.5 | 15.2 | 9.7 | |||||
| 103 | 104 | 1.20 ±0.29 | 1.13 ±0.22 | 18.1 | 18.8 | 12.2 | 17.8 | 10.8 | 8.5 | ||
| 2010 | 170 | 11.97 ±1.98 | 1.33 ± 0.23 | 15.8 | 11.6 | 4.4 | |||||
| 80 | 90 | 1.37 ±0.24 | 1.29 ±0.22 | 12 | 18.7 | 6.1 | 15.7 | 2 | 6.3 | ||
| 2016 | 138 | 10.23 ±1.51 | 1.24 ±0.20 | 21.8 | 21.7 | 2.5 | |||||
| 62 | 76 | 1.23 ±0.20 | 1.26 ±0.21 | 20 | 25.5 | 20.7 | 22.2 | 5.7 | 0 | ||
a Goitre incidence (age-adjusted) according to the reference values proposed by Zimmermann et al. [14]
b goitre incidence (BSA-adjusted) according to the reference values proposed by Zimmermann et al. [14]
c goitre incidence (age-adjusted) according to the reference values proposed by Szybiński et al. [15].
The study from 1994 was part of an international survey on iodine deficiency in Europe (Thyromobil Project) [7], while that from 1999 was part of a nationwide survey on the effectiveness of iodine prophylaxis in Poland [8]. Studies in 2010 [9] and 2016 were carried out within the project carried out by the Polish Mother’s Memorial Hospital – Research Institute in Lodz. All studies were performed by employees of the same endocrinology department.
The Ethics Committee at the Polish Mother’s Memorial Hospital – Research Institute, Lodz, Poland (No. 64/2016) approved the protocol for each study. Parents of all the children qualified for the experiment gave written consent for participation of their children in the study. The Ethics Committee approved the protocol.
Anthropometric data
The height and the body mass of the children were measured using standard anthropometric techniques [10]. Children were measured without shoes and in light indoor clothing (each survey was performed at the beginning of the summer).
The heights were measured to the nearest millimetre with a Harpenden stadiometer, and the weights of children were recorded to the nearest 100 g. Body surface area (BSA) was calculated from the following formula: Wt 0.425 × H 0.725 × 71.84 × 10–4, where: Wt – weight (kg), H – height (cm).
Urine iodine concentration (UIC)
Urine samples were collected from each child, prior to the physical examination. Samples were stored at –70°C until assay. In order to determine UIC, the modified catalytic method by Sandell and Kolthoff was used [11].
Thyroid volume (V)
Thyroid physical examination was performed, followed by ultrasound examination of the thyroid gland with a Siemens Sonoline SI-400 (1994 and 1999), Siemens Sonoline Prima (2010) and SonoScape S6 (2016) devices with 7.5 MHz linear array transducers. The examinations were carried out by two experienced ultrasound examiners (endocrinologists with many years of experience in performing thyroid ultrasound). The examination was performed in a supine position (the child was lying down on a medical coach). The sum of lateral thyroid lobe volumes (determined sonographically) constituted the actual volume of the thyroid gland (V); the volume of the isthmus was not included. The volume of the thyroid lobe was calculated according to the following formula, proposed by Brunn et al. [12]: V (× 10–6 m3) = 0.479 × W × D × L, where W = width (cm), D = depth (cm), L = length (cm). We compared the volume of the thyroid gland V (m3) to BSA (m2) by calculating V/BSA ratio (m × 10–6). This ratio was proposed and used by us in our previous studies [9, 13]. The obtained data were also compared to reference values for thyroid volume proposed by Zimmermann et al., adjusted for age and for BSA [14], as well as to reference values proposed by Szybiński et al., adjusted for age [15]. Only children aged 7–12 years and with BSA between 0.7 and 1.6 m2 were included in the analysis, as the standards were proposed only for those who met these criteria.
Statistical analysis
The data were statistically analysed using the nonparametric test for independent groups (Mann-Whitney rank sum test), Kruskal-Wallis one-way analysis of variance on ranks, followed by the Dunn test and χ2 analysis. In all analyses, statistical significance was accepted at the level of p < 0.05. Data processing, statistical analyses, and figures were performed using SigmaPlot 12.3 (Systat Software, Inc., San Jose, CA, USA) and Excel (Microsoft Corp., Redmond, WA, USA).
Results
Urine iodine concentration
The median UICs were 45.5 μg/l (1994), 101.1 μg/l (1999), 100.6 μg/l (2010) and 288.3 μg/l (2016). The median value of UICs in 1994 was significantly lower in comparison to the other values. The median value of UICs in 2016 was significantly higher than the other values. The median values of UICs from 1999 and 2010 did not differ. The median UIC values are presented in Figure 1.
Figure 1
Urinary iodine concentration (UIC) in the examined children. Upper and lower limits of boxes are 75th and 25th percentiles, respectively. Horizontal dashed line and solid line in the boxes represent the median and mean values, respectively. Whiskers indicate standard deviation (SD). The horizontal red dotted line indicates a value of 100 μg/l. Points represent a scatter of UIC results
ap < 0.05 vs. 1999, 2010 and 2016; bp < 0.05 vs. 1999 and 2010.
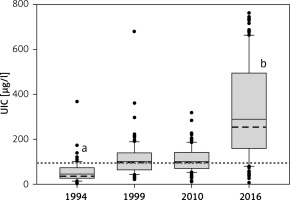
There were no statistically significant differences between the UIC median values in the group of girls and boys at each time point, and the values were, respectively, in 1994, 50.3 μg/l vs. 39.2 μg/l; in 1999, 104.5 μg/l vs. 97.3 μg/l; in 2010, 100.1 μg/l vs. 105.9 μg/l; and in 2016, 285.3 μg/l vs. 307.2 μg/l.
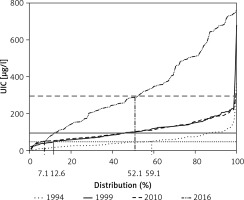
The distribution of UIC is shown in Figure 2. The proportions of children, expressed as a percentage, in whom UIC was lower than 50 μg/l were: in 1994, 59.1%; in 1999, 12.6%; in 2010, 7.1%; and in 2016, 7.1%. The proportions of children whose UIC was higher than 300 μg/l were in 1994, 1.1%; in 1999, 1.0%; in 2010, 0.6%; and in 2016, 47.9%.
Figure 2
Distribution of urinary iodine concentration (UIC) in the examined children. Dotted line, solid line, dashed line and dashed-dotted line are distributions of UIC in years 1994, 1999, 2010 and 2016, respectively. The horizontal solid line, the horizontal dash line and the horizontal dotted line are values of 100 μg/l, 300 μg/l and 50 μg/l of UIC, respectively. The percentage of children with UIC < 50 μg/l: 59.1% (1994); 12.6% (1999); 7.1% (2010, 2016), as well as children with UIC > 300 μg/l – 52.1% (2016) is presented on the graph
Thyroid volume
The size of the thyroid assessed by ultrasonography and presented as the V/BSA ratio was 6.55 × 10–6 m in 1994 and was higher than the V/BSA ratios at the next three time points, which were: in 1999, 2.73 × 10–6 m; in 2010, 2.73 × 10–6 m; and in 2016, 2.70 × 10–6 m. Figure 3 shows the V/BSA values at particular time points with respect to sex.
Figure 3
Ratio of thyroid volume (V) [× 10–6 m3] to body surface area (BSA) [m2] in examined children – V/BSA
Whiskers indicate standard deviation (SD). ap < 0.05 vs. 1999, 2010 and 2016; bp < 0.05 vs. boys in 2010.
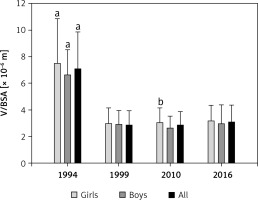
It was found that V/BSA in girls was higher than in boys only in 2010 (2.93 × 10–6 m vs. 2.59 × 10–6 m). At the remaining time points, V/BSA differences between girls and boys were not statistically significant and were: in 1994, 6.76 × 10–6 m vs. 6.36 × 10–6 m; in 1999, 2.87 × 10–6 m vs. 2.71 × 10–6 m; in 2016, 2.78 × 10–6 m vs. 2.68 × 10–6 m.
The goitre incidence calculated on the basis of the reference values proposed by Zimmermann et al. [14] was the highest in 1994 and was 92.6% and 95.4% (age- and BSA-adjusted, respectively) and was lower at the other time points: in 1999, 18.5% and 15.2%; in 2010, 15.8% and 11.6%; and in 2016, 21.8 and 21.7%.
The goitre incidence, calculated on the basis of the reference values proposed by Szybiński et al. [15], was also the highest in 1994 and was 66.6% (in girls and boys 75% and 59% respectively); in 1999, 9.7% (8.5% and 10.8%); in 2010, 4.4% (6.3% and 2%); and in 2016, 2.5% (0 and 5.7%). The values of goitre incidence are presented in Table I.
Discussion
The present results show that after introduction of iodine prophylaxis, the iodine intake in the studied population increased continuously. The value of median UIC in 1994 (45.5 μg/l) corresponded to moderate iodine deficiency, while median values after iodine prophylaxis (1999 – 101.1 μg/l; 2010 – 100.6 μg/l; 2016 – 288.3 μg/l) corresponded to adequate values; however, the last value (2016) was markedly higher than the previous two, being close to the upper limit of UIC accepted as normal [16]. The obtained results are consistent with other studies evaluating the supply of iodine in Poland [17, 18]. Thus, Poland has become a country where iodine intake should be considered sufficient [19].
Although the median values of UICs in 1999 and 2010 do not differ, the distribution of iodine supply has changed for the better (the percentage of UIC < 50 μg/l dropped from 12.1% in 1999 to 7.1% in 2010). These data not only prove the effectiveness of iodine prophylaxis, but they also show a more homogeneous increase in iodine supply in the studied population. The analysis of the prevalence of goitre is also evidence of longer-lasting, increased iodine supply. While in 1999 the percentage of goitre was 9.7% according to the reference values defined by Szybiński et al. [15] (which corresponded to mild iodine deficiency), the analysis of goitre incidence at subsequent time points proved that goitre was no longer considered endemic (4.4% and 2.5% in 2010 and 2016, respectively). The obtained data indicate that drawing conclusions on the iodine supply, based only on median UIC values, is insufficient, especially during the transition period, i.e. after the introduction of a new iodine prophylaxis model. During this period, the diet of many people remains almost the same as before, so it may take several months or even years until the iodine supply improves.
While the use of the reference values proposed by Szybiński et al. [15]. correlates relatively well with the iodine supply in the studied population, the goitre analysis according to the reference values proposed by Zimmermann et al. [14], under the auspices of the WHO, is not a useful tool. Results obtained in this way (Table I) erroneously suggest that there is still goitre endemicity in the study population. The fact that the reference data were developed based on examinations carried out in a supine position may be the possible cause of this inconsistency [14, 20, 21]. Such a discrepancy may also result from different methods of measurement and associated inter-observer and intra-observer errors [20].
Use of the V/BSA ratio is a much better method for analysing changes in thyroid size [13]. The V/BSA value was the highest in 1994 and decreased significantly after the introduction of iodine prophylaxis. There were no statistically significant differences between 1999 and 2010, and 2016 (despite statistically significant differences among the UIC values). This proves that only iodine deficiency (1994) leads to enlargement of the thyroid gland and consequently to goitre formation. The adequate iodine supplies, varying either around the minimum normal values (median UIC: 101.1 μg/l in 1999; 100.6 μg/l in 2010), or around the maximum normal values (median UIC: 288.3 μg/l in 2016), do not have a significant effect on thyroid size. It should be stressed that the last, highest value (2016) was associated with no iodine-induced adverse signs or symptoms.
Our present data concerning UICs in school-aged children in 2016, which probably correspond to a markedly increased iodine intake, are quite surprising. It is worth noting that in 2016 the proportion of children with UIC above 300 μg/l increased significantly – 47.9% vs. 0.6% (2010), 1.0% (1999) and 1.1% (1994). It is difficult to explain these findings exclusively by an increase in consumption of iodized salt in the population. On the contrary, for many years in Poland, as in other countries, multidirectional measures to reduce dietary salt intake have been observed [22]. The average salt intake in Poland at the time of the introduction of iodine prophylaxis was 15 g/day [23], whereas currently the goal is to reduce the intake of NaCl in the general population to less than 5 g/day [24]. Such systematic reduction of salt intake as an iodine carrier has raised fears that Poland, particularly in the face of iodine supply being close to the minimum normal values (1999, 2010), could again become a country with iodine deficiency [25]. The reappearance of iodine deficiency has, in the past, already affected several countries, such as Australia [26]. On the other hand, the results of our studies (2016) are consistent with the results of studies on iodine supply in the Slovak population (median of UIC was 140–320 μg/l depending on the study group) [27], whose iodine prophylaxis is based on a model very similar to the Polish one (obligatory consumption of iodized salt, containing 25 mg KI/kg of NaCl or equivalent iodine value in the form of KIO3), and the type of diet in both countries is also similar. The diversification of iodine sources may be the possible explanation of the obtained results. At the time of introduction of iodine prophylaxis, there were few alternative sources of iodine. At present, drinking water containing iodine, most commonly at a concentration of 0.1–0.2 mg/l, is being consumed more and more frequently [25].
The relatively narrow range of potential intake is an advantage of salt as an iodine carrier, since salt as a spice is only an additive to consumed food. For other basic iodine-containing food products (including water, milk or meat), the range of their potential intake increases significantly, which may lead to iodine intake at doses much higher than recommended (e.g. consuming a large amount of drinking water containing iodine during hot days and/or during physical activity).
Interestingly, some of the medical mineral waters contain more iodine (e.g. Franciszek water deriving from the spa town of Wysowa-Zdroj (southern Poland close to the border with Slovakia) contains 2.9 mg of I/l, and Henryk water from the same spa town contains 0.9 mg of I/l) [28]. Consuming them according to the manufacturer’s general recommendations (200 ml 2–3 times a day), for prevention of various diseases, corresponds to 1,160–1,740 μg of I/day. In the case of medical recommendations, water consumption can be even greater. However, it seems very unlikely that the potential consumption of the above-mentioned mineral waters could be considered as a reason for the UIC values obtained by us in 2016, because the distribution of these waters is markedly limited in Poland not only due to high iodine anion concentrations but also because of very high content of sodium cations.
Another example of additional sources of iodine in Poland is the iodination of animal food, which consequently results in the phenomenon that milk, milk products and meat are becoming year by year richer sources of iodine for humans [23]. It is worth adding that some products containing iodine (e.g. mineral drinking water) are actively advertised as “healthy food”, and in the Polish population there is still a belief that Poland is a country of iodine deficiency (hence many people choose the seaside as a destination of their vacation, thus increasing iodine intake with inhaled air).
The processes of integration between countries and the free flow of people and goods are also of great importance for iodine supply in the population. Food (especially imported by individuals) does not need to meet the standards of iodine content set by a country, because models of iodine prophylaxis usually differ in different countries.
In conclusion, it may be concluded that iodine prophylaxis is not an event, but a process that changes over time; thus the key to the permanent elimination of iodine deficiency is the monitoring of this process and its modification, depending on the changing conditions.
The iodine prophylaxis introduced in 1997 has proved to be effective in eliminating iodine deficiency in the studied population, which, together with the data from other studies, shows that Poland has become a country with an adequate iodine supply. The diversification of iodine sources, despite the reduction of salt consumption, has led to an increase in iodine supply in the population of school-aged children, expressed as the UICs close to the upper limit of values considered normal. Further increase in iodine supply, particularly uncontrolled, may be unfavourable for health; therefore constant and active monitoring of iodine prophylaxis is required to ensure optimum iodine intake.


