Introduction
Chemotherapy is a pivotal part of cancer treatment and chemotherapy usage has been gradually increased in the world for this reason. It is known that chemotherapeutic agents damage ovaries and may cause ovarian failure and infertility [1]. Due to these damaging effects of chemotherapy on ovarian reserve, many prophylactic agents have been used to try to protect against destructive effects of chemotherapies on ovarian reserve [2].
Cisplatin was the most commonly used alkylating agent in gynecological cancers. Most of the studies revealed that GnRH agonist or antagonist usage with chemotherapeutic agents may protect ovarian reserve [3, 4]. Alkylating agents may affect ovaries with cortical fibrosis, blood vessel damage, and severe reduction in primordial follicles and ovarian cell proliferation. Also arrest and apoptosis may start in granulosa cells.
The fertility of women may be determined by the ovarian reserve tests including counting the number of ovarian follicles and measuring the level of anti-Mullerian hormone (AMH). The tests are the most widely used parameters in infertility clinics [5].
To date, there has been no study clearly investigating ovarian reserve with anti-Müllerian hormone (AMH) and histological analysis after cisplatin exposure with a GnRH agonist or antagonist in an animal model. In this prospective randomized controlled trial, we evaluated the effects of a GnRH agonist or antagonist on ovarian reserve of rats exposed to cisplatin, which was the most commonly used chemotherapeutic agent in gynecological cancers.
Material and methods
Twenty-four five- to six-month-old female Wistar-Albino rats weighing 180–210 g were used in this study. All procedures were approved by Ethics Committee of Kobay Experimental Animal Laboratory. All procedures were carried out at Kobay Experimental Animal Laboratory. All rats were housed under controlled temperatures (22 ±2°C) and 12/12 h light/dark cycle with food and water ad libitum.
Twenty-four Wistar albino rats were randomly divided into three groups, each consisting of eight rats. In the GnRH agonist group (group 1), rats received a single dose of 50 mg/m2 cisplatin with 1 mg/kg Triptorelin. In the GnRH antagonist group (group 2), rats were received single dose 50 mg/m2 cisplatin with 1 mg/kg Cetrorelix. In the control group (group 3), rats received 50 mg/m2 cisplatin.
Blood samples (2 ml) were collected from the heart of the rats before laparotomy. The serum of the blood was separated from cells by centrifugation, and samples were frozen at –20°C until assayed. AMH levels (ng/ml) were determined by using ELISA (Cusabio Rat AMH kit; Biotek Synergy HT Microplate Reader, US) in Duzen Laboratory by a researcher blinded to the groups. All samples were tested in the same assay. The coefficients of intra- and inter- assay variations were 4.8% and 7.5%, respectively.
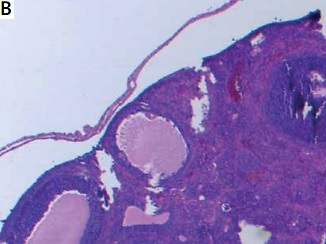
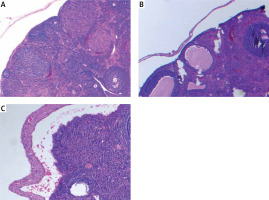
Ovarian samples were randomly prepared in 5-μm slices to assess follicles [6]. Hematoxylin-eosin was used for staining of the samples. The same pathologist blindly examined the ovarian follicles and noted the counts of the follicles. Primordial, primary, secondary and tertiary follicles were defined according to the usual standard textbooks of microscopic anatomy [6].
Results
Ovarian reserve was assessed by AMH and histology. There was no difference in the primordial follicle count between the groups. Primary follicle counts were higher in group 2 (4.50 ±1.47 vs. 3.50 ±1.70 vs. 3.00 ±3.54) and secondary follicle counts were higher in group 1 (2.96 ±1.11 vs. 1.74 ±1.03 vs. 1.37 ±3.11). Tertiary follicle counts were higher both in group 2 and 3 compared to the control group (1.36 ±0.83 vs. 0.84 ±0.99 vs. 0.50 ±0.75). The total follicle count of the study groups was significantly higher compared with the control group (14.32 ±5.96 vs. 12.48 ±4.12 vs. 10.63 ±6.80). AMH was found to be significantly higher in groups 1 and 2 compared with the control group (18.56 ±25.33 vs. 16.48 ±24.66 vs. 9.37 ±26.54). Histological examination of the groups is demonstrated in Figure 1. There is no difference between the protective effect of GnRH agonist and GnRH antagonist (Table I).
Table I
Results of ovarian follicle tests and endometrium thickness of the groups
Discussion
Fertility and premature menopause are major concerns for young women who are undergoing treatment for cancer. Premature ovarian failure caused by chemotherapy depends on type and dosage of the agents. Alkylating agents such as cisplatin are widely used chemotherapeutic agents and cause ovarian damage. Cisplatin has a heavy metal combination and antineoplastic effects on rapidly divided cells [7]. In this prospective randomized study, we evaluated how to decrease the detrimental effect of cisplatin on ovaries.
There are lots of experimental and clinical studies on protective agents such as gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists [8–10]. Our previous study showed that a GnRH agonist with cisplatin may protect ovarian reserve compared to solely cisplatin [11]. Controversially after this study meta-analyses showed that GnRH agonists had no effect to protect ovarian reserve against chemotherapy [12]. Then a committee opinion suggested that the data on the use of GnRH analogs for ovarian suppression have been conflicting in terms of the efficacy, so other fertility preservation options should be offered in addition to GnRH analog treatment [13]. But the studies and meta-analyses evaluated different types and dosages of chemotherapeutic agents. Additionally application of GnRH analogs was different. In our study we added a GnRH analog on the same day as cisplatin.
The protective mechanism of the GnRH agonists on the ovaries after exposure to cytotoxic effects on ovaries involves the GnRH receptors at the gonadal and pituitary levels. A GnRH agonist may downregulate the receptors of gonads and suppress primordial follicle development [14]. Additionally, the hypoestrogenic state decreases ovarian perfusion and delivery of chemotherapy to the ovaries and a direct effect of the GnRH agonist on the ovary occurs independently of the gonadotropin level may be discussed for the protection of ovarian germ cells [15, 16]. Our study showed that GnRH agonist administration has a protective effect on ovarian reserve compared with the control group.
Recently, GnRH antagonists have been used to decrease the effects of chemotherapy on ovaries [17, 18]. A GnRH antagonist shows higher competitive blockade of the receptors than a GnRH agonist. Additionally a GnRH antagonist inhibits apoptosis triggered by chemotherapy [17]. To our knowledge, there has been no study comparing the protective effects of a GnRH agonist and antagonist on ovaries exposed to chemotherapy. Our study was the first to show the effects of a GnRH agonist and antagonist on ovaries after application of cisplatin, which was the most commonly used chemotherapeutic agent in gynecological malignancies.
Various ovarian reserve tests have been used to predict the ovarian response and pregnancy outcomes in couples. Two of most widely used tests are AMH levels and AFC [19, 20]. AMH is an increasingly used reliable marker of present ovarian reserve and the number of follicles remaining in ovaries [21]. In this study, ovarian reserve tests such as AMH and histological follicles count were used.
This study clearly demonstrated that cisplatin had a cytotoxic effect on the ovaries and combination with a GnRH agonist and antagonist had statistically significant protective effects on the total follicle count but not the primordial follicles. Although primordial follicles did not significantly change, primary, secondary and tertiary follicles might be protected with a GnRH agonist and antagonist. Additionally, AMH levels supported this situation and increased with the GnRH agonist and antagonist.
In conclusion, ovarian reserve was first evaluated after exposure to cisplatin with a GnRH agonist and antagonist in an animal randomized prospective study using most common ovarian reserve tests including AMH and histological examination of ovarian follicles. These data represent an important benefit for understanding the effect of GnRH agonists and antagonists on ovarian reserve. Also, both GnRH agonists and antagonists have limited protective effects on ovarian reserve.