Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
GASTROENTEROLOGY / CLINICAL RESEARCH
Evaluation of differences between initial and recurrent acute pancreatitis in the intensive care unit
1
Department of Emergency, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
2
Division of Critical Care, Nanxiang Hospital of Jiading District, Shanghai, China
3
Department of Geriatrics, Medical Center on Aging, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
4
International Laboratory in Hematology and Cancer, Shanghai Jiao Tong University School of Medicine/Ruijin Hospital/CNRS/Inserm/Côte d’Azur University, Shanghai, China
5
The State Key Laboratory of Medical Genomics, Pôle Sino-Français de Recherche en Sciences Du Vivant et Génomique, Shanghai, China
These authors had equal contribution to this work
Submission date: 2024-04-02
Final revision date: 2024-08-27
Acceptance date: 2024-08-28
Online publication date: 2024-09-06
Corresponding author
Wei Xu
Department of Emergency, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 197 Ruijin 2nd Road, Huangpu District, 200025, shanghai, China
Department of Emergency, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 197 Ruijin 2nd Road, Huangpu District, 200025, shanghai, China
KEYWORDS
intensive care unitrecurrent pancreatitisacute pancreatitismortalityMedical Information Mart for Intensive Care-IV
TOPICS
ABSTRACT
Introduction:
Previous studies have found that patients with recurrent acute pancreatitis (RAP) may be at reduced risk for a clinically severe course and have reduced mortality. However, there is still a lack of data related to RAP patients admitted to the intensive care unit (ICU).
Material and methods:
Baseline characteristics of patients diagnosed with initial and recurrent acute pancreatitis from the Medical Information Mart for Intensive Care/MIMIC-IV database were extracted. In-hospital mortality and length of hospital/ICU stay were identified as outcomes. Binomial logistic regression analysis was performed to clarify the independent risk factors for in-hospital mortality in both groups, and we determined the best scoring system for prognosis prediction by plotting the receiver operating characteristic (ROC) curves and the decision curve analysis (DCA) curves.
Results:
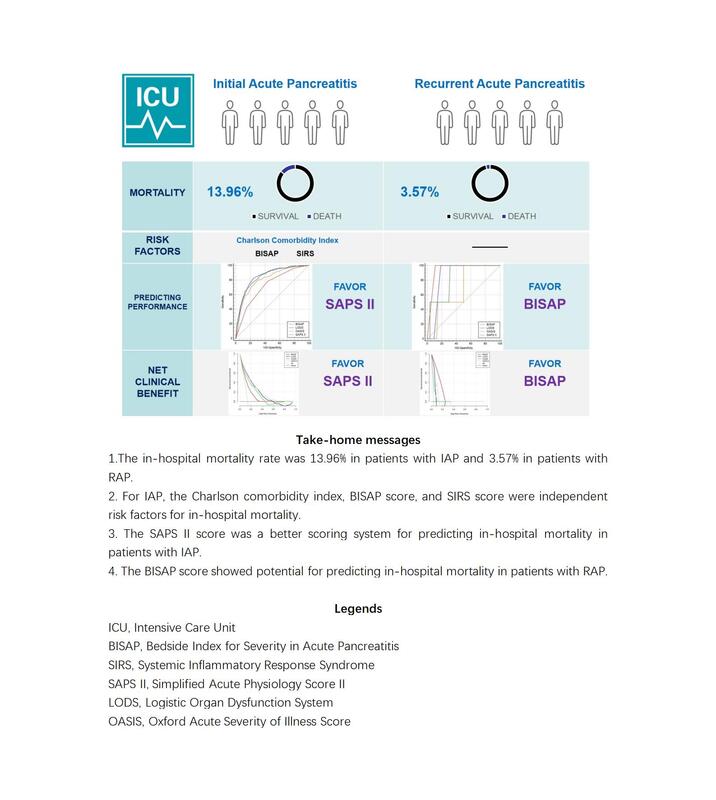
The in-hospital mortality rate was 13.96% in patients with initial acute pancreatitis (IAP) and 3.57% in patients with RAP, and there was no statistically significant difference between the two groups regarding length of hospital/ICU stay. For IAP, the Charlson Comorbidity Index, the Bedside Index for Severity in Acute Pancreatitis (BISAP) score, and the Systemic Inflammatory Response Syndrome (SIRS) score on the first day of admission were independent risk factors for in-hospital mortality. Age, gender, Charlson Comorbidity Index, BISAP score, SIRS score, and obesity were not independent risk factors for in-hospital mortality in patients with RAP. For patients with IAP, the areas under the ROC curves (AUCs) of the four scoring systems (the BISAP, the Logistic Organ Dysfunction System (LODS), the Oxford Acute Severity of Illness Score (OASIS), and the Simplified Acute Physiology Score II (SAPS II)) were 0.720, 0.847, 0.808, and 0.845, respectively, but the results of the Z test showed no significant difference between LODS and SAPS II; The DCA showed that at the threshold of 0.2–0.6, SAPS II score almost always showed a higher net clinical benefit than the other scoring systems, but when the threshold exceeded 0.6, none of the four scoring systems showed a net clinical benefit. For patients with RAP, the AUCs of the four scoring systems (BISAP, LODS, OASIS, and SAPS II) were 0.944, 0.861, 0.681, and 0.829, respectively, but the AUC value of BISAP was only significantly different from that of LODS; the DCA showed that in the threshold range of 0–0.25, BISAP score almost always showed a higher net clinical benefit than the other scoring systems, but in other threshold ranges, none of the four scoring systems showed a net clinical benefit.
Conclusions:
RAP is less severe and has a lower risk of in-hospital mortality than IAP. The Charlson Comorbidity Index, the BISAP, and the SIRS score on the first day of admission were all independent risk factors for in-hospital mortality in patients with IAP. The SAPS II score was a better scoring system for predicting in-hospital mortality in patients with IAP. In contrast, the BISAP score showed potential for predicting in-hospital mortality in patients with RAP.
Previous studies have found that patients with recurrent acute pancreatitis (RAP) may be at reduced risk for a clinically severe course and have reduced mortality. However, there is still a lack of data related to RAP patients admitted to the intensive care unit (ICU).
Material and methods:
Baseline characteristics of patients diagnosed with initial and recurrent acute pancreatitis from the Medical Information Mart for Intensive Care/MIMIC-IV database were extracted. In-hospital mortality and length of hospital/ICU stay were identified as outcomes. Binomial logistic regression analysis was performed to clarify the independent risk factors for in-hospital mortality in both groups, and we determined the best scoring system for prognosis prediction by plotting the receiver operating characteristic (ROC) curves and the decision curve analysis (DCA) curves.
Results:
The in-hospital mortality rate was 13.96% in patients with initial acute pancreatitis (IAP) and 3.57% in patients with RAP, and there was no statistically significant difference between the two groups regarding length of hospital/ICU stay. For IAP, the Charlson Comorbidity Index, the Bedside Index for Severity in Acute Pancreatitis (BISAP) score, and the Systemic Inflammatory Response Syndrome (SIRS) score on the first day of admission were independent risk factors for in-hospital mortality. Age, gender, Charlson Comorbidity Index, BISAP score, SIRS score, and obesity were not independent risk factors for in-hospital mortality in patients with RAP. For patients with IAP, the areas under the ROC curves (AUCs) of the four scoring systems (the BISAP, the Logistic Organ Dysfunction System (LODS), the Oxford Acute Severity of Illness Score (OASIS), and the Simplified Acute Physiology Score II (SAPS II)) were 0.720, 0.847, 0.808, and 0.845, respectively, but the results of the Z test showed no significant difference between LODS and SAPS II; The DCA showed that at the threshold of 0.2–0.6, SAPS II score almost always showed a higher net clinical benefit than the other scoring systems, but when the threshold exceeded 0.6, none of the four scoring systems showed a net clinical benefit. For patients with RAP, the AUCs of the four scoring systems (BISAP, LODS, OASIS, and SAPS II) were 0.944, 0.861, 0.681, and 0.829, respectively, but the AUC value of BISAP was only significantly different from that of LODS; the DCA showed that in the threshold range of 0–0.25, BISAP score almost always showed a higher net clinical benefit than the other scoring systems, but in other threshold ranges, none of the four scoring systems showed a net clinical benefit.
Conclusions:
RAP is less severe and has a lower risk of in-hospital mortality than IAP. The Charlson Comorbidity Index, the BISAP, and the SIRS score on the first day of admission were all independent risk factors for in-hospital mortality in patients with IAP. The SAPS II score was a better scoring system for predicting in-hospital mortality in patients with IAP. In contrast, the BISAP score showed potential for predicting in-hospital mortality in patients with RAP.
REFERENCES (37)
1.
Ingraham NE, King S, Proper J, et al. Morbidity and mortality trends of pancreatitis: an observational study. Surg Infect (Larchmt) 2021; 22: 1021-30.
2.
Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep 2009; 11: 97-103.
3.
Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet 2020; 396: 726-34.
4.
Lee PJ, Bhatt A, Holmes J et al. Decreased severity in recurrent versus initial episodes of acute pancreatitis. Pancreas 2015; 44: 896-900.
5.
Sun Y, Jin J, Zhu A, et al. Risk factors for recurrent pancreatitis after first episode of acute pancreatitis. Int J Gen Med 2022; 15: 1319-28.
6.
Zhang W, Shan HC, Gu Y. Recurrent acute pancreatitis and its relative factors. World J Gastroenterol 2005; 11: 3002-4.
7.
Tao H, Xu J, Li N, et al. Early identification of high-risk patients with recurrent acute pancreatitis progression to chronic pancreatitis. Arch Med Sci 2022; 18: 535-9.
8.
Sadr-Azodi O, Oskarsson V, Discacciati A, et al. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol 2018; 113: 1711-9.
9.
Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016; 3: 160035.
10.
Le Gall JR, Klar J, Lemeshow S et al. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA 1996; 276: 802-10.
11.
Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med 2013; 41: 1711-8.
12.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270: 2957-63.
13.
Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57: 1698-703.
14.
Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010; 105: 435-41; quiz 442.
15.
Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis – a prospective observational study. Int J Surg 2018; 54: 76-81.
16.
Gao W, Yang HX, Ma CE. The value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta-analysis. PLoS One 2015; 10: e0130412.
17.
Lee PJW, Stevens T. Reply to: Yang et al, Clinical features of recurrent acute pancreatitis: experience from a single center. Pancreas 2017; 46: e37-8.
18.
Mallick B, Shrama DJ, Siddappa P, et al. Differences between the outcome of recurrent acute pancreatitis and acute pancreatitis. JGH Open 2018; 2: 134-8.
19.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373-83.
20.
Longnecker DS. Role of the necrosis-fibrosis sequence in the pathogenesis of alcoholic chronic pancreatitis. Gastroenterology 1996; 111: 258-9.
21.
Acharya C, Cline RA, Jaligama D, et al. Fibrosis reduces severity of acute-on-chronic pancreatitis in humans. Gastroenterology 2013; 145: 466-75.
22.
Huang S, Ma J, Dai H, Luo L. A new in-hospital mortality prediction nomogram for intensive care unit patients with acute pancreatitis. Arch Med Sci 2024; 20: 61-70.
23.
Wang L, Zhang Z, Hu T. Effectiveness of LODS, OASIS, and SAPS II to predict in-hospital mortality for intensive care patients with ST elevation myocardial infarction. Sci Rep 2021; 11: 23887.
24.
Chang X, Pan J, Zhao R, et al. DDOST correlated with malignancies and immune microenvironment in gliomas. Front Immunol 2022; 13: 917014.
25.
Chen S, Gao C, Du Q, et al. A prognostic model for elderly patients with squamous non-small cell lung cancer: a population-based study. J Transl Med 2020; 18: 436.
26.
Hu T, Zhang Z, Jiang Y. Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: a retrospective propensity score matching analysis. Clin Chim Acta 2021; 521: 272-7.
27.
Hou X, Wang D, Zuo J, et al. Development and validation of a prognostic nomogram for HIV/AIDS patients who underwent antiretroviral therapy: data from a China population-based cohort. EBioMedicine 2019; 48: 414-24.
28.
Darvas K, Futó J, Okrös I, et al. [Principles of intensive care in severe acute pancreatitis in 2008]. Orv Hetil 2008; 149: 2211-20.
29.
Jaber S, Garnier M, Asehnoune K, et al. Guidelines for the management of patients with severe acute pancreatitis, 2021. Anaesth Crit Care Pain Med 2022; 41: 101060.
30.
Pelli H, Sand J, Laippala P, Nordback I. Long-term follow-up after the first episode of acute alcoholic pancreatitis: time course and risk factors for recurrence. Scand J Gastroenterol 2000; 35: 552-5.
31.
Pelli H, Lappalainen-Lehto R, Piironen A, et al. Risk factors for recurrent acute alcohol-associated pancreatitis: a prospective analysis. Scand J Gastroenterol 2008; 43: 614-21.
32.
Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol 2012; 107: 1096-103.
33.
Rebours V, Vullierme MP, Hentic O, et al. Smoking and the course of recurrent acute and chronic alcoholic pancreatitis: a dose-dependent relationship. Pancreas 2012; 41: 1219-24.
34.
Seppänen H, Puolakkainen P. Classification, severity assessment, and prevention of recurrences in acute pancreatitis. Scand J Surg 2020; 109: 53-8.
35.
Räty S, Pulkkinen J, Nordback I, et al. Can laparoscopic cholecystectomy prevent recurrent idiopathic acute pancreatitis? A prospective randomized multicenter trial. Ann Surg 2015; 262: 736-41.
36.
Testoni PA. Acute recurrent pancreatitis: etiopathogenesis, diagnosis and treatment. World J Gastroenterol 2014; 20: 16891-901.
37.
Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 2019; 16: 175-84.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.