Introduction
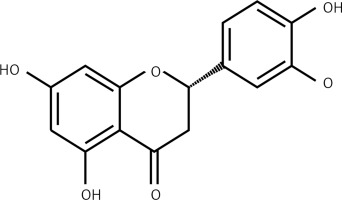
Flavonoids are commonly present across the plant kingdom and have been reported to exhibit tremendous pharmacological potential. A diversity of medicinal plants showing the presence of high amounts of flavonoids have been used in several traditional systems of medicine for the treatment of several diseases and disorders. The medicinal properties of these plants are mainly due to the presence of these secondary metabolites [1, 2]. Among natural products, flavonoids represent an important part of the human diet, and in the United States the estimated regular dietary intake of mixed flavonoids is up to 1 g. This figure may be even higher for people improving their diets with flavonoid-rich herbal preparations [3]. With advances in medical research, flavonoids are being evaluated for their diverse bioactivities. So far they have been reported to exhibit a wide range of activities – anti-inflammatory, estrogenic, enzyme inhibition, antimicrobial, anti-allergic, antioxidant, antitumor and others [4]. Owing to their fairly consistent structure, flavonoids impede the activity of a wide range of eukaryotic enzymes and therefore exhibit diversity of activities. The different parts of the flavonoid molecules have been considered critical for their bioactivities [4]. The present study was therefore designed to evaluate the anticancer potential of eriodictyol (Figure 1) against the human lung cancer cell line A549 and to decipher the underlying mechanism. Lung cancer is the major cause of cancer related deaths in the world and in China [5, 6]. The severe increase in the incidence of cancers, the dearth of suitable cures and the severe side effects accompanying the synthetic drugs have made it necessary to explore new and more effective molecules. With the upsurge in the incidence of drug resistance, the treatment and management of cancers has become even more difficult [5, 6]. In the current study eriodictyol was evaluated against human lung cancer cells and it was found to exhibit an IC50 of 50 µM. The results of the present study indicated that eriodictyol exhibits significant anticancer activity by inducing apoptosis in human lung cancer A549 cancer cells, altering mitochondrial membrane potential (MMP) and causing cell cycle arrest via downregulation of the expression of Bcl-2 and upregulation of Bax expression. In conclusion, we propose that eriodictyol may prove to be a potential candidate for the development of anticancer chemotherapy for lung cancer.
Material and methods
Chemical reagents, cell lines and culture conditions
All chemicals and reagents including eriodictyol (98% pure by HPLC) were obtained from Sigma-Aldrich. Human lung cancer (A549) and non-cancerous human epithelial FR2 cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin and maintained in a humidified atmosphere containing 5% CO2. All of the reagents were procured from Hyclone (Logan, UT, USA).
Anti-proliferative assay
MTT was used to determine the anti-proliferative activity of eriodictyol against the human lung cancer A549 cells [7]. The A549 cells in 100 μl of culture medium were seeded in a 96-well plate at a density of 3 × 103 cells/ml and kept at 37°C in 5% CO2 for 48 h. After 24 h, an additional 100 μl of complete medium with either no additions or different concentrations (0–100 µM) of eriodictyol was added. Thereafter, the cells were incubated for 12, 24, and 48 h. This was followed by the addition of 20 μl of MTT solution (5 mg/ml) and incubation for 4 h. Afterwards the medium was removed and 150 μl of DMSO added. The absorbance (OD) of each well was measured at 490 nm using a Tunable Mi-185 croplate Reader (EL-x 800, BioTek Instruments, USA).
Determination of apoptotic populations and DNA damage
Human A549 cells at a density of 2 × 105 cells/well were seeded in 6-well plates and then administered with 0, 25, 50 and 100 µM eriodictyol for 48 h. For estimation of apoptotic cell populations an FITC-Annexin V/PI Apoptosis detection kit was used following the manufacturer’s instructions (Beijing Biosea Biotechnology, China). The alkaline comet assay was carried out to evaluate DNA damage efficacy of eriodictyol as described previously [8, 9].
Cell cycle analysis
For cell cycle analysis the A549 cells were treated with 0, 25, 50 and 100 µM eriodictyol concentration of KAM and the percentage of cells in each of the cell cycle phases was estimated using the Muse Cell Analyzer and Muse Cell Cycle Kit according to the manufacturer’s protocol (Merck Millipore).
Mitochondrial membrane potential (MMP)
Human lung cancer A549 cells were seeded at a density of 2 × 105 cells/well in a 6-well plate and kept for 24 h and treated with 0, 25, 50 and 100 µM of the test compound for 48 h at 37°C in 5% CO2 and 95% air. Afterwards, the cells from all treatments were collected, washed twice with PBS and re-suspended in 500 µl of DOC6 (1 µmol/l) for MMP at 37°C in a dark room for 30 min. The samples were then analyzed instantly using flow cytometry.
Western blotting analysis
After administration with various concentrations of eriodictyol, cells were harvested and lysed in lysis buffer (20 mM HEPES, 350 mM NaCl, 20% glycerol, 1% Nonidet P 40, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, protease inhibitor cocktail, and phosphatase inhibitor cocktail). Out of the total protein samples a 20 μg aliquot was separated on 10% SDS-PAGE gel. The gel was then transferred to nitrocellulose membranes, blocked with 5% BSA and probed with a primary antibody. This was followed by probing with the required secondary antibody. Finally, the signal was perceived with WEST-SAVE Up luminal-based ECL reagent (ABFrontier, Korea).
Results
Anticancer activity of eriodictyol on A549 human lung cancer cells
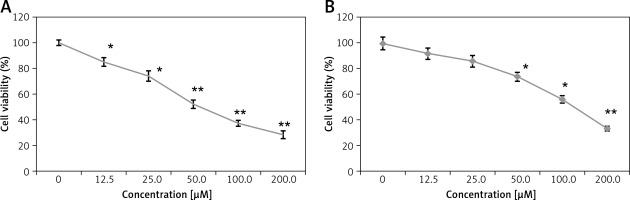
Anticancer activity of eriodictyol (Figure 1) was evaluated against human A549 cancer and non-cancerous FR2 cells. The MTT assay at 0–100 µM concentration showed that eriodictyol exhibited a concentration-dependent activity. The IC50 of eriodictyol against human A549 cells was found to be IC50 50 µM (Figure 2 A) as against the IC50 of 95 µM against the noncancerous FR2 cells (Figure 2 B).
Eriodictyol caused apoptosis and DNA damage in human A549 lung cancer cells
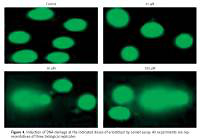
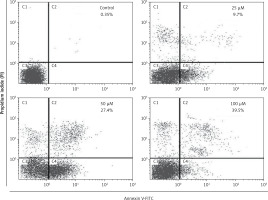
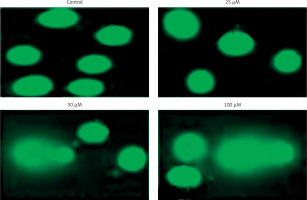
In order to confirm apoptotic cell death induced by eriodictyol annexin V/PI staining was carried out at the concentrations of 0, 25, 50 and 100 µM. Flow cytometric results showed that the percentage of apoptotic cell population increased to 9.7%, 27.4% and 39.5% in human A549 cancer cells after 48 h at the concentrations of 25, 50 and 100 µM, respectively, as compared to the untreated control (Figure 3). Thus the results indicate that eriodictyol caused apoptotic cell death of human A549 cancer in a concentration-dependent manner. Furthermore, the results of the comet assay showed that eriodictyol caused DNA damage in A549 human lung cancer cells dose dependably (Figure 4) and the DANA damage increased with the increasing concentration of the drug.
G2/M phase arrest of A549 cancer cells triggered by eriodictyol
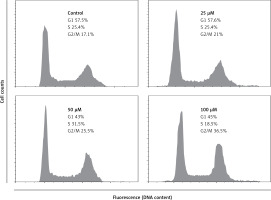
Our results indicated that eriodictyol caused G2/M cell cycle arrest in a dose. It was observed that the percentage of cells was considerably increased in G2/M at the concentrations of 0 to 100 µM of eriodictyol, causing G2/M arrest (Figure 5). Additionally, the populations of A549 cells in G2/M phase were only slightly elevated at a dose of 25 µM. However, the apoptotic cell populations significantly increased at G2/M phase at the concentration of 50 µM. Moreover, the eriodictyol induced G2/M phase increase of A549 cancer cells showed a dose-dependent trend.
MMP loss in human A549 lung cancer cells
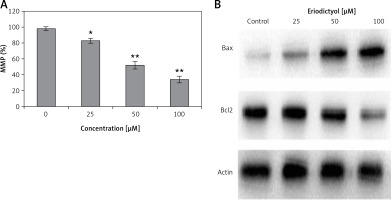
Cells were administered with 0, 25, 50 and 100 µM eriodictyol for various time periods and the levels MMP were evaluated. A significant reduction of MMP level (Figure 6 A) was observed in the treated A549 cells as compared to the control. At concentrations of 0, 25, 50 and 100 µM, the MMP was found to be 81, 52 and 33% as compared to the untreated human A549 cancer cells.
Effect of eriodictyol on Bcl-2/Bax signalling pathway
To evaluate whether eriodictyol could induce apoptosis, the expression of pro-apoptotic proteins Bcl-2/Bax was evaluated using western blot assay. The findings are shown in Figure 6 B and indicate an interesting outcome. The increased Bax/Bcl-2 ratio causes activation of caspase 3 and hence apoptosis. In our results, compared to the untreated control cells, eriodictyol-treated cells showed concentration-dependent downregulation of Bcl-2 and upregulation of Bax proteins, hence increasing the Bax/Bcl-2 ratio and ultimately inducing apoptosis.
Effect of eriodictyol on m-TOR/PI3K/Akt signalling pathway
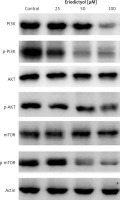
Whether eriodictyol could modulate the protein expression of the m-TOR/PI3K/Akt signalling pathway was evaluated using western blot analysis. The findings are shown in Figure 7 and indicate an interesting outcome. As compared to the untreated control cells, eriodictyol-treated cells showed concentration-dependent downregulation of m-TOR and pm-TOR proteins. It also caused downregulation of PI3K/Akt protein expression levels.
Discussion
Lung cancer is one of the deadly cancers occurring around the globe. Hundreds of thousands of patients are diagnosed with this disease annually [5]. Although existing treatment options exhibit decent clinical results, still hundreds of thousands of cancer-related deaths are attributed to lung cancer. Moreover, existing treatment options have severe side effects which badly influence the quality of life [6]. Nonetheless, development of drug resistance has made cancer very difficult to treat. Therefore natural products are known to have multiple targets and are able to suppress or activate complicated signalling pathways to inhibit cancer cell growth [7]. In the current study, eriodictyol showed potential growth-inhibiting activity against human lung A549 cancer cells, as was evident from the proliferation assay. Eriodictyol exhibited an IC50 of 50 µM against the human A549 cells as compared to the IC50 of 95 µM against the noncancerous FR2 cells, clearly indicating that eriodictyol is less toxic to normal cells. Several studies have reported that flavonoids are not toxic to normal cells and may therefore prove to be potential anticancer agents [9]. Flavonoids such as eriodictyol are regularly consumed in the human diet and are present at high concentrations in fruits, vegetables and other plant-derived foods, such as teas and other beverages and hence are considered non-toxic [10].
As reported previously, many drugs exhibit antiproliferative effects via induction of apoptosis. Several existing drugs have been reported to alter explicit apoptotic signalling pathways [9–14]. Additionally, drug resistance is partially explained by the potential of cancer cells to escape apoptosis [15]. It was observed that eriodictyol induces apoptosis in a concentration-dependent manner. Mitochondrial outer membrane permeability (MOMP) is an important process involved in the apoptotic pathway. In the present study it was observed that eriodictyol showed a significant reduction in the MMP and exhibited a concentration-dependent pattern. Our results are consistent with studies carried out earlier [16]. Therefore the results suggest that eriodictyol may induce apoptosis by cumulative intracellular reduction of MMP. It has been reported that many anti-cancer drugs target cancer cells partially by causing reduction of MMP [17]. Analysis of apoptotic cell populations revealed that eriodictyol induces apoptosis and DNA damage in human lung cancer cells, as demonstrated by the comet assay. These observations are in agreement with previous studies wherein natural products such as isoliquiritigenin were found to inhibit cancer cell growth and induce apoptosis [18]. Flow cytometry using propidium iodide as a probe was used to study the effects of eriodictyol on cell cycle progression. Eriodictyol induced G2/M cell cycle arrest and led to a significant increase of G2/M cells dose dependently. Our results are consistent with previous studies wherein natural products have been reported to inhibit cell proliferation and induce cycle arrest in a dose-dependent manner [19]. Additionally, effects of eriodictyol on Bcl-2/Bax signalling were studied using western blot assay. The results showed eriodictyol acid-treated cells showed concentration-dependent downregulation of Bcl-2 and upregulation of Bax proteins, ultimately inducing apoptosis. Members of the Bcl-2 family proteins, such as Bcl-2, Bax and Bak, are believed to play key controlling roles in the execution of cell apoptosis. However, several studies have shown that Bcl-2 family proteins also aim at the apoptotic pathway [19, 20]. Finally, the effect of eriodictyol on the PI3/AKT/mToR signalling pathway was investigated and it was observed that it caused inhibition of a key protein of the pathway. These results are interesting since this target is considered important cancer chemotherapy.
Taken together, our results have shown that eriodictyol is a potent anti-cancerous molecule. Furthermore, flavonoids are generally non-toxic and can hence be used at higher concentrations in humans. The results of the present study pave the way for further evaluation of this molecule against more cell lines and under in vivo conditions.
In conclusion, in the present study, our data provide a framework for the construction of cell death pathways in A549 cells in response to eriodictyol by inducing apoptosis, regulating the Bcl-2/Bax signalling pathway and mainly by inhibition of the PI3K/AKT/m-TOR signalling pathway. All of these contribute to the inhibition of growth of cancer cells, and eriodictyol might potentially serve as a potential candidate for cancer therapy.