Introduction
Cytokines are involved in immune homeostasis and regulation of the inflammatory response. They promote effective control of pathogens, enforce tolerogenic mechanisms and improve tissue regeneration after injury [1]. Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are important hematopoietic growth factors with major roles associated with the production and development of granulocytes, monocytes and other antigen-presenting cells [2]. Granulocyte colony-stimulating factor and GM-CSF are also pleiotropic growth factors affecting various functions of immune system cells, e.g. modulation of chemotaxis and migration of neutrophils, phagocytosis of neutrophils and monocytes, surface expression of Fc- and complement-mediated cell-binding (FcγR1, CR-1, CR-3 and adhesion receptor) or modulation of inflammatory reaction [3]. Importantly, G-CSF and GM-CSF participate in the regeneration of injured tissues. It has been shown that the cytokines are involved in the proliferation and migration of endothelial cells and in the development of blood vessels [4, 5], the proliferation of keratinocytes [6], fibroblasts and myofibroblasts [7], as well as in collagen synthesis [8].
The wide effects of cytokines on the organism suggest that G-CSF and GM-CSF can be used for the treatment of various diseases. Cytokine therapies are widely used in clinical practice. Cytokine administration increases the number of neutrophils in the peripheral blood and increases the number of peripheral blood stem cells [9]. Granulocyte colony-stimulating factor therapy is successfully used in oncology for the treatment of several advanced neoplasms. Granulocyte colony-stimulating factor or GM-CSF therapies are used for in the following treatments: (1) to treat patients with advanced metastatic disease after chemotherapy to treat or prevent neutropenia, and also in bone marrow transplantation to help to recover from the bone marrow depression induced by chemotherapy [10]; (2) in conjunction with chemotherapy to treat patients with advanced breast carcinoma [11]; (3) to promote the acceleration of myeloid recovery in patients with non-Hodgkin’s lymphoma, acute lymphoblastic leukemia, and Hodgkin’s disease undergoing autologous stem cell transplantation [2]; (4) to treat brain tumor cells [11]; (5) to treat autoimmune diseases such as inflammatory bowel disease (IBD), Crohn disease and ulcerative colitis [12]; (6) to accelerate myeloid recovery in patients undergoing stem cell transplantation [2]. Granulocyte colony-stimulating factor is also used to decrease the incidence of infection in patients with non-myeloid malignancies [2].
However, systematic administration of G-CSF and GM-CSF cytokines can produce significant changes in the bone marrow and peripheral blood WBCs. The effects of G-CSF and GM-CSF on peripheral blood neutrophils include toxic granulation, increased level of granulocytes and their vacuolation, and abnormalities of nuclear segmentation. Other less common features include binucleate promyelocytes and myelocytes, giant myeloid precursors, and rarely, the development of bone marrow fibrosis [9]. Granulocyte-macrophage colony-stimulating factor may also cause monocytosis and eosinophilia. Some trials using very high doses of GM-CSF were often associated with adverse effects such as pericarditis and thrombosis [13]. In order to avoid toxic side-effects associated with systemic cytokine administration, cytokines can be encapsulated within the liposomes and delivered locally. Liposomes are vesicles composed of one or more lipid membranes surrounding an aqueous lumen. Liposomal formulations have been used to encapsulate and deliver various therapeutics, such as small molecules, proteins and oligonucleotides [1]. The use of liposomes improves the biological activity of encapsulated compounds by protecting them from cell metabolism and inactivation in the plasma, increasing their half-life or by improving their tissue-specific biodistribution [14, 15].
The main aim of the present work was to characterize and examine the effect of G-CSF and GM-CSF loaded liposomes on mesenchymal stem cells (isolated from the umbilical cord stem cells [UCSC]) and cord blood hematopoietic stem cells (CBHSC). The cells used in this study are well-defined primary stem cells lines, which perform various functions in the body. The UCSC are mesenchymal stem cells, which exhibit the capacity to differentiate into mesodermal (osteocytes, adipocytes, and chondrocytes), ectodermal (neurocytes) and endodermal (hepatocytes) lineages [16]. The UCSC express cell surface markers such as CD29, CD73, CD90, CD105 and do not express CD14, CD34, CD45 and HLA (human leucocyte antigen)-DR [17]. The CBHSC are hematopoietic stem cells, which are able to differentiate into hematopoietic stem cell populations (white or red blood cells and platelets) [18]. The CBHSC express cell surface markers such as CD34, HLA-DR and c-kit tyrosine kinase receptor [19]. Because our previous studies showed that negatively charged liposomes have the lowest cytotoxicity and do not affect hematopoietic cell proliferation [20] we chose this type of liposomes for our present study.
Material and methods
Stem cells
Mesenchymal stromal cell
Umbilical cord stem cells were isolated as previously described [21]. Briefly, umbilical cord fragments were isolated after childbirth (the protocol was accepted by the local Ethical Committee, permit no. KB 70/2012). Fragments of Wharton jelly were transferred to the culture flask (75 cm2, BD Falcon, Poland) and maintained in growth medium (DMEM with GlutMax, 20% of FBS and 50 UI/ml of antibiotics: penicillin/streptomycin; all from Gibco, Poland). Cells were passaged 3–5 times before use. After the 3rd passage, the cells were phenotyped for the presence of mesenchymal stem cell markers CD29, CD34, CD45, CD73, CD90, CD105, CD106 (all antibodies were purchased from BD, Poland).
All markers were analyzed by flow cytometry (FACS Caliber, BD, USA) as previously described [21]. 95% of cells exhibited strong expression of CD29 and CD90; high CD73 and CD105; week expression of CD106 and lack of expression of CD34 and CD45 markers, which is consistent with the results described in the literature [17].
Results of flow cytometry analysis are presented in Supplementary Figure S1.
Cord blood hematopoietic stem cells
Cord blood hematopoietic stem cells were isolated as previously described [11]. Cord blood samples were collected during delivery with the mothers’ consent (the protocol was accepted by the local Ethical Committee). The mononuclear cells were isolated by Ficoll-Uropoline sedimentation (Stem Cell Technologies, UK) and frozen in liquid nitrogen (–170°C) until further use.
Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor
Lyophilized G-CSF and GM-CSF cytokines were purchased from Peprotech IMC (USA). Cytokines were dissolved in phosphate-buffered saline (PBS, Gibco, Poland) with 0.1% human albumin (Octapharma, Poland) in the concentration of 100 ng/ml, aliquoted and stored at –80°C until use.
Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor concentration analysis
Granulocyte colony-stimulating factor or GM-CSF concentration in all test was analyzed by an ELISA kit (R&D Systems, USA) according to the manufacturer’s protocol. To obtain the values within a detection range of ELISA kits the G-CSF samples were diluted 1 : 4 and the GM-CSF samples were diluted 1 : 20. Each sample in ELISA analysis was added in duplicate.
Production of granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor loaded liposomes
Liposomes were produced by the hand-shaking method [22]. Due to the previously observed strong cytotoxic effect of positive-charge and neutral-charge liposomes on all types of tested cells, only negative-charge liposomes were selected for cytokine encapsulation experiments [11].
According to the previously developed protocol, one dose of cytokine encapsulated in the liposomes was composed of: L-α-phosphatidylcholine (100 mg, Sigma Aldrich, Poland, cat. no. P3556), cholesterol (5.6 mg, Sigma Aldrich, Poland, cat. no. C8667) and dihexadecyl phosphate (8.0 mg, Sigma Aldrich, Poland, cat. no. D2631). Reagents were suspended in 15 ml of chloroform (Sigma Aldrich, Poland) in a 250-ml round-bottom flask, which was rotary evaporated (R-300 Rotavapor, Büchi, Poland, 60 RPM) in a water bath at 30°C to obtain a thin dry film. The residual solvent was removed by vacuum evaporation for 1 h. The only modification of the previously used method was the addition of 1 ml of G-CSF or GM-CSF stock solution (100 ng/ml) dissolved in 10 ml of PBS with 0.1% human albumin. The flask was rotated in an evaporator at 60 rpm for 30 min at room temperature. All tests on liposomes were performed after overnight incubation (4°C). To avoid contamination from the cytokines not encapsulated in the liposomes, the cytokine-loaded liposome suspension was centrifuged at 10,000 g for 1.5 h, the supernatant was removed and the pellet was resuspended in PBS. This procedure was repeated four times.
Supernatants from the last centrifugation were collected, and the absence of free G-CSF or GM-CSF was confirmed by ELISA analysis (data not shown).
Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor content in the liposomes
Efficiency of cytokine encapsulation
Cytokine concentration was measured with ELISA tests (R&D system, USA). To evaluate the efficiency of G-CSF or GM-CSF encapsulation in the liposomes we assessed cytokine concentration in (1) fresh defrosted cytokine solution (control), (2) liposome-containing PBS/albumin “opened” or not by treatment (20 min at room temperature) with 5% Tween 20, (3) liposomes after centrifugation “opened” by treatment (20 min at room temperature) with 5% Tween 20, (4) supernatant after liposome centrifugation. To assess the possible effect of Tween 20 (Sigma Aldrich, Poland) the Tween was also added to the control samples. The results are presented as mean ± standard deviation (SD). All samples were analyzed in sextuplets.
In order to check if we can produce the nanoliposomes with encapsulated cytokines, the liposome-containing PBS/albumin solution was homogenized (15 ml of the solution, per 5 min) in EmulsiFlex C3 machine (Avestin, Australia). Then cytokine concentrations of homogenized liposomes were analyzed in sextuplets.
Efficiency of cytokine encapsulation in liposomes of various sizes
The minimum diameter of liposomes encapsulating chemical particles depends on many parameters and cannot be predicted theoretically [23]. Therefore G-CSF or GM-CSF prepared liposomes were filtered through polycarbonate filters (Millipore, Poland), with pore size 200 nm, 400 nm, and 600 nm. The concentration of cytokines encapsulated in ≤ 200 nm, ≤ 400 nm, and ≤ 600 nm diameter liposomes was analyzed using an ELISA kit. Results are presented as the % of the whole liposome population ± SD. All samples were analyzed in sextuplets.
Biological activity of liposome encapsulated cytokines (granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor)
Cytotoxic effect of liposomes on umbilical cord stem cells growth
The cytotoxic effect of cytokine-loaded liposomes on UCSC growth was evaluated by proliferation assay. UCSC cells were seeded in 24-well culture plates (BD Falcon, Poland) in a concentration of 2 × 104 cells per well in DMEM with GlutaMAX, 20% of FBS with 50 UI/ml of penicillin/streptomycin. Following 24 h of incubation, the liposome suspension (liposomes in PBS supplemented with 0.1% of albumin) was added. Liposome suspension accounted for 5% of the total volume of culture medium, i.e. about 0.38 μg of liposomes/μl. After 5 days of culture, the proliferation assay was performed. We used 4 independent tests: cell number count (Bürker chamber), MTT assay, according to Rokicki et al. [24], NR-assay and SRB assay, according to Lewicki et al. [20]. Each assay was performed in triplicate (n = 6). The results are presented as the percentage of control values (mean ± SD).
Effect of liposome encapsulated granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on cord blood hematopoietic stem cells growth
Granulocyte colony-stimulating factor or GM-CSF-loaded liposomes were used as cytokine source in in vitro clonal cultures of hematopoietic cells (CBHSC). Cord blood hematopoietic stem cells cells were cultured in 24-well plates (1 × 104 cells per well) in commercial methylcellulose medium. Two different methylcellulose media were used (all from Stem Cell Technologies, UK): H4230 without growth factors and without Epo, and H4330 without growth factors and with Epo. 50 μl of liposome suspension (loaded with G-CSF or GM-CSF) were added to 950 μl of methylcellulose medium and mixed thoroughly prior to cell addition. Before liposome addition, the concentration of encapsulated cytokines was evaluated. For each study, approximately 10 ng/ml of G-CSF or GM-CSF added to 50 µl of liposome solution was used. The positive control consisted of 50 µl of pure cytokines in PBS with 0.1% human albumin (at concentration 10 ng/ml). Controls consisted of 50 µl of PBS with 0.1% human albumin (without cytokines, C1) or 50 µl of “empty” liposome suspension. The number of cells (formed by mixed cells Mix-CFCs, erythroid cells – BFU-Es or granulocyte-macrophage cells GM-CFCs) was analyzed after 14 days of culture. Results were presented as a percentage of control cells’ (C1) values at 14 days of culture. All experiments were performed in triplicate (n = 6). The results are presented as the percentage of control values (mean ± SD).
Storage effect
We evaluated the effect of storage temperature of G-CSF- or GM-CSF-loaded liposomes on the activity of cytokines extracted from liposomes. We used three common storage temperatures: –20oC, +4oC and +24oC. Cytokine content was determined at the beginning of the experiment, and subsequently at the 1st, 7th, 14th, and 28th day of storage. The cytotoxic effect of the prepared liposomes (cell number, MTT, NR, and SRB assay) was assessed as described above. The results are presented as the mean ± standard deviation. All samples were analyzed in sextuplets.
Statistical analysis
Statistical evaluation of the results was performed using the t-test and one-way ANOVA with Bonferroni correction (in the case of a normal distribution) or non-parametric Kruskal-Wallis and Mann-Whitney U tests (in the case of an abnormal distribution). Assessment of the distribution of the data was evaluated using the Shapiro-Wilk test. GraphPad Prism software was used to carry out these tests (version 7; GraphPad Software, Inc., La Jolla, CA, USA). P < 0.05 was considered a statistically significant difference.
Results
Efficiency of cytokine encapsulation
The analysis of the efficiency of encapsulation of G-CSF and GM-CSF is presented in Table I. The deduction of the value of cytokine concentration in PBS from the value of the concentration after cytokine release to PBS by opening liposomes with Tween 20 represented the amount of cytokine encapsulated in the liposomes. Results of the analyses showed that 12–13% of the total amount of cytokines was successfully encapsulated. We are aware that this is not very high encapsulation efficiency in comparison to the efficiencies obtained in other studies [25, 26]; however, our protocol is relatively simple and does not contain the post-encapsulation steps, such as the freeze-thawing cycles, which increase cytokine concentration in liposomes. Homogenized liposomes contained less than 3% of encapsulated cytokines, and therefore were not used in further analysis.
Table I
Granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) concentration in phosphate-buffered saline presented as concentration in pg/ml and % of the control value (fresh defrosted G-CSF or GM-CSF solution)
Effect of liposome size on the concentration of encapsulated cytokines
Nearly 50% of cytokines (GM-CSF – 47.4% and G-CSF – 42.9%) were encapsulated in ≤ 600 nm liposomes, while only 32% of GM-CSF and 28.3% of G-CSF were encapsulated in 200–400 nm liposomes. The ≤ 200 nm liposomes contained less than 20% of total amounts of cytokines (G-CSF – 17.6% and GM-CSF – 15.3%). The analysis of the percentage of cytokines encapsulated in different size liposomes is summarized in Table II.
Biological activity of cytokines (granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor) encapsulated in liposomes
Effect of liposomes on umbilical cord stem cells growth
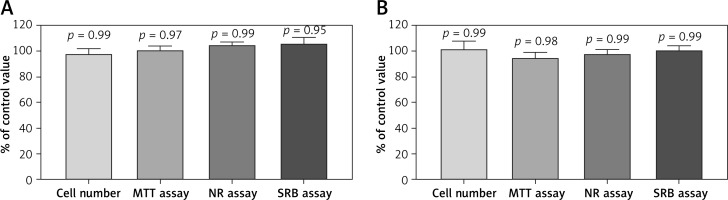
The addition of freshly prepared G-CSF or GM-CSF-loaded liposomes into the cultures of UCSC cells did not result in any change in cell morphology. We also did not observe any change in the proliferation and cell death in comparison to the control cells (Figure 1).
Figure 1
Effect of A – granulocyte colony-stimulating factor (G-CSF) or B – granulocyte-macrophage colony-stimulating factor (GM-CSF) loaded liposomes on proliferation of umbilical cord stem cells cells as assessed by cell number, MTT, NR and SRB assays. Assays were performed in triplicate (n = 6). Results are shown as a mean percentage ± SD of control values. P – probability value, statistical significance p < 0.05
Effect of liposomes on cord blood hematopoietic stem cells growth
Methylcellulose medium without erythropoietin
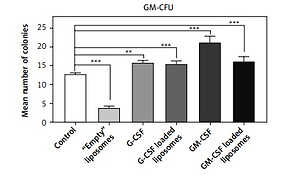
Cord blood hematopoietic stem cells cultured in methylcellulose medium without Epo and without growth factors (H4230, Stem Cell Technologies) differentiated only into granulocyte-macrophage cell colonies (GM-CFU). Co-culture of CBHSC cells with G-CSF-loaded liposomes or soluble G-CSF significantly increased the number of cells after 14 days of incubation in H4230 methylcellulose medium in comparison to the control conditions (cells without cytokine treatment) by 21% – p < 0.01 or 23%, respectively p < 0.001). Also, the soluble fraction of GM-CSF-loaded liposomes and pure GM-CSF increased the number of cells (126% and 166% respectively, p < 0.001). The increased number of colonies after treatment with GM-CSF-loaded liposomes was however lower than after treatment with soluble GM-CSF and the difference was statistically significant (p = 0.032). The empty liposomes strongly reduced cell proliferation in control conditions (30%, p < 0.001) (Figure 2 A).
Figure 2
The effect of liposome-encapsulated granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) cytokines on hematopoietic stem cell (CBHSC) proliferation. Liposomes (at final concentration 5%) were added to the H4230 methylcellulose medium without Epo (Stem Cell Technologies) (A) or to the H4330 methylcellulose medium without growth factors supplemented with Epo (B). Results are presented as number of granulocyte/macrophages (GM) (A) or BFU-E, Mix-CFU and GM-CU colonies (B). Results are shown as a mean ± SEM. Assays were performed in triplicate (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001

Methylcellulose medium with erythropoietin
Cord blood hematopoietic stem cells cultured in methylcellulose medium containing Epo without growth factors (H4330) differentiated into three types of colonies: mixed cells (Mix-CFU), erythroid cells (BFU-E), or granulocyte-macrophage cells (GM-CFU). Co-culture of CBHSC cells with cytokine-loaded liposomes significantly (p > 0.001) increased the number of Mix-CFU and GM-CFU colonies after 14 days of incubation. The liposome loaded with cytokines did not affect the growth of BFU-E cells in comparison to the control (Figure 2 B).
Storage effect
Concentration of granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor
Although it is known that storage below –70°C or lyophilization guarantees the unlimited lifetime of proteins [27], the typical storage conditions for the drugs and wound dressings are –20°C (freezer), +4°C (fridge) or +24°C (room temperature). Using the ELISA test, we analyzed the effect of different storage temperatures on the concentration of the active form of G-CSF and GM-CSF encapsulated in the liposomes, which were suspended in PBS with albumin. The measurements were performed at weekly intervals within 28 days of storage.
In the control condition (PBS with G-CSF) the storage at 24°C caused a fast decrease of concentration of G-CSF (at the 28th day only about 45% of cytokine was found by ELISA). At 4°C, 49% and at –20°C – 75% of cytokine was found at the 28th day of the experiment. Encapsulation of GM-CSF in liposomes resulted in a slower reduction of G-CSF during 4°C and 24°C storage (59% and 54% respectively). Interestingly, freezing at –20°C resulted in the preservation of approximately 90% of G-CSF at day 28, which was about 15% higher than in the control. A summary of these results is presented in Figure 3 A.
Figure 3
The effect of temperature and time of storage on granulocyte colony-stimulating factor (G-CSF) (A) or granulocyte-macrophage colony-stimulating factor (GM-CSF) (B) loaded liposomes. The concentration of cytokines extracted from liposomes was analyzed by ELISA test. Cytokine concentration was tested at the beginning of the experiment, and subsequently at the 1st, 7th, 14th, and 28th day of storage. Comparison between control solution and cytokine-loaded liposomes. P – probability value, statistical significance p < 0.05
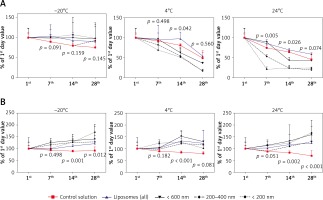
In contrast to the relatively low G-CSF stability, the GM-CSF was much more stable at all tested temperatures (Figure 3 B). At 24°C the concentration of cytokine was about 70%, at 4°C about 79% and at –20°C about 91% of fresh cytokine concentration. Liposome encapsulation of GM-CSF caused increased stability of GM-CSF concentration as compared to the fresh cytokine at the 28th day of the experiment.
Cytotoxic effect on umbilical cord stem cells growth
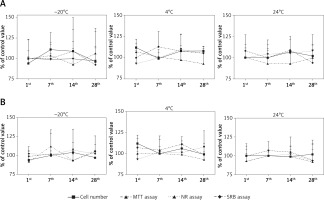
The addition of G-CSF- or GM-CSF-loaded liposomes to the UCSC cells did not affect cell morphology, number or mortality (Figures 4 A, B). We did not observe any effect on UCSC proliferation when the soluble or liposome encapsulated G- or GM-CSF cytokines were used (data not shown).
Figure 4
The effect of storage temperature of granulocyte colony-stimulating factor (G-CSF) (A) or granulocytemacrophage colony-stimulating factor (GM-CSF) (B) loaded liposomes on proliferation measured by cell count, MTT, NR and SRB assay. Liposomes were tested at the 1st, 7th, 14th, and 28th days of storage in differ temperatures
Discussion
Liposomes are a well-known method of cytokine delivery to the cells used in treatments of many infectious diseases [28]. Liposomes offer several advantages over conventional delivery systems, especially for the parenteral administration (i.e. intravenous and intraperitoneal injection) [29]. They are targeted to the specific cell types and the delayed release of cytokine from the liposomes results in sustained paracrine-like delivery of the cytokines. The most important role of liposomes, however, is to protect the cytokines or other encapsulated molecules from the biodegradation [29]. The encapsulation in liposomes results in different biodistribution, and improves the pharmacokinetics, biological activity in vivo, and the therapeutic efficiency in comparison to the non-encapsulated drugs [23]. The limitation of liposome methodology is a potential reduction of bioavailability of the drug, saturation of the immune competent cells with lipids, and potentially increased toxicity of some drugs due to their enhanced interactions with particular cells. The benefits and limitations of liposome drug carriers critically depend on the type of interactions of liposomes with cells and their fate in the body [30].
Anderson et al. [31] showed that the liposome composition is important and can be tailored to deliver bioactive cytokines such as IL-1α, IL-2, IL-6, IFN-γ or GM-CSF to their target locations. In order to address the limitations and therapeutic potential of encapsulated cytokines, the cytokine release rate, the time of residence at the injection site, the biodistribution and bioactivity have to be understood [1]. Here we have developed an efficient protocol for the liposome encapsulation of GM-CSF and G-CSF cytokines. The encapsulated cytokines are stable and active for a long period of time. The GM-CSF cytokine is clinically approved for the acceleration of myeloid recovery in patients with non-Hodgkin’s lymphoma, acute lymphoblastic leukemia, and Hodgkin’s disease undergoing autologous stem cell transplantation. GM-CSF is used for neutropenia associated with stem cell transplantation. The G-CSF cytokine is used for the treatment of neutropenia and for the mobilization of peripheral hematopoietic progenitor cells for stem cell transplantation [2].
The physicochemical parameters of liposomes, such as size, shape, and charge, affect bio-distribution, pharmacokinetics, and life span of encapsulated cytokines [32]. Kedar et al. [25] suggested that liposome-encapsulated GM-CSF and TNF-α might be more efficient immunomodulators than the soluble cytokines. Our protocol allows for encapsulation of 12–13% of fresh cytokines in the liposomes. In other studies, the encapsulation level of proteins was shown to be between 10% and 95% and depended on several factors such as the interaction between the protein and phospholipid bilayer, protein mass or electric charge of liposomes [33, 34]. Although 12–13% is not very high encapsulation efficiency, we obtained liposomes which are nontoxic to the cells and contain biologically active cytokines, efficiently taken up by the cells, which was confirmed by CBHSC assay. The encapsulation efficiency can be easily increased for example by using several freeze-thawing cycles [35]. The positive effect of freeze-thawing may explain a slight increase of GM-CSF concentration, which we observed in the liposomes stored at –20°C. We showed that over 50% of encapsulated cytokines is entrapped in ≤ 600 nm liposomes. A similar result, about 50–60% of encapsulation efficiency for G-CSF ≤ 600 nm liposomes, was obtained by Kiafar et al. [26] for DDPC/cholesterol liposomes prepared by thin layer film hydration.
The major limitation of the liposome delivery system is the instability of the load. For this reason, we examined the effect of storage conditions on the concentration of liposome-encapsulated cytokines. We observed that storage at 24°C caused a fast decrease of G-CSF concentration in the liposomes, and freeze-storage at –20°C resulted in the preservation of 90% of G-CSF concentration for 28 days. It has been shown that the reduced temperature storage from 20°C to 4°C not only improves the stability of liposomes [36] but also prolongs the viability of biologically active compounds [37]. In contrast to the relatively low G-CSF stability, the GM-CSF was fully stable for 28 days when stored at all temperatures tested. Biological activity of G-CSF/GM-CSF encapsulated in the liposomes was tested on UCSC and CBHSC cells. We did not observe any change in the morphology or cell number in the samples treated with cytokines-loaded liposomes (G-CSF or GM-CSF) for the period of at least 1 month, after –20, +4 and 24°C storage.
The most important question of the study was whether the cytokines encapsulated in liposomes remain biologically active and are able to stimulate specific processes in cells after being released from the liposomes. To test this we used umbilical cord hematopoietic stem cells (CBHSC), which are known to respond to G-CSF or GM-CSF supplementation by increasing the number of colonies in the methylcellulose culture. The co-culture of CBHSC cells with G-CSF or GM-CSF-loaded liposomes significantly increased the number of cells after 14 days of incubation in H4230 methylcellulose medium in comparison to the control conditions (21% – p < 0.01 for G-CSF-loaded liposomes and 26% p < 0.001 for GM-CSF loaded liposomes). A similar result (increased number of colonies) was obtained for fresh cytokines: 23%, p < 0.001 and 66%, p < 0.001 respectively for G-CSF and GM-CSF. Significant differences in the number of GM-CFU colonies (about 30%, p = 0.032) between the soluble cytokine- and liposome-loaded GM-CSF samples may be the result of increased affinity of the cytokine to the liposome structure. This may also in part explain the higher level of the GM-CSF found in the liposomes after the 14th and 28th day of storage. Interestingly, the empty liposomes strongly reduced cell colony number, about 70% (p < 0.001) in comparison to the control (Figure 2 A). The results concerning empty liposomes agree with our previous study, in which addition of liposomes to methylcellulose medium limited in growth factors (H4230) caused a significant reduction of the number of GM-CFU colonies [11]. In contrast, the addition of empty liposomes to methylcellulose medium supplemented with growth factors did not affect GM-CFU colonies. In this study, we also observed this relationship. Empty liposomes caused a reduction of GM-CFU also in other methylcellulose medium used in the study (H4330 methylcellulose medium –limited in growth factors, but with the addition of erythropoietin). However, the addition of cytokines to liposomes eliminated the adverse effects of empty liposomes. The number of colonies in liposome-loaded cytokines was almost similar to that observed after fresh cytokine addition. We did not observe effects of the liposome-loaded cytokines on the number of BFU-E and Mix-CFU colonies.
Taking the evidence together, both cytokines studied here (G-CSF and GM-CSF) encapsulated in liposomes were biologically active and did not cause side effects in the stem cells after prolonged storage. This indicated that we developed an efficient method for cytokine encapsulation. However, before this technology can be applied therapeutically the efficiency of cytokines encapsulation has to be improved.
In conclusion, the presented method allows efficient encapsulation of G-CSF and GM-CSF in liposomes. In all tested temperatures of storage, the cytokines (especially GM-CSF) were more stable when encapsulated in liposomes than in the control solution. The cytokines were released from liposomes and caused a biological effect in the CBHSC culture. These results are very promising for the future use of G-CSF and GM-CSF loaded liposomes in preclinical trials.