Epicardial adipose tissue (EAT), the visceral fat surrounding the heart, has recently emerged as a significant player in the pathophysiology of cardiovascular diseases (CVD) [1]. EAT is not just a storage site for energy, but is rather an active endocrine and paracrine organ that secretes several bioactive molecules, collectively referred to as adipocytokines [2]. These adipocytokines have been implicated in the development and progression of atherosclerosis, insulin resistance, and other CVD risk factors [3]. Statins, widely prescribed for their lipid-lowering properties despite the introduction of newer lipid-lowering agents [4–6], have shown remarkable pleiotropic effects beyond their primary cholesterol-lowering capabilities [7–12]. These effects encompass anti-inflammatory, antioxidant, anti-thrombotic, and endothelial function-improving properties [7–14]. Given the growing recognition of EAT as a relevant contributor to CVD [15], investigating the impact of statin therapy on EAT characteristics has become a topic of great interest. Understanding the influence of statin therapy on EAT holds significant clinical implications [16]. By modulating the properties of EAT, statins may potentially attenuate the adverse effects of EAT-derived adipocytokines on the cardiovascular system [17]. Moreover, considering the anatomical proximity of EAT to the coronary arteries, statin-induced changes in EAT may have direct implications for the development and progression of coronary artery disease [11, 18, 19]. To comprehensively evaluate the impact of statin therapy on EAT, a systematic review and meta-analysis are warranted. Such an analysis can consolidate the existing evidence, assess the consistency of findings across studies, and provide a quantitative estimation of the effect size.
Therefore, this study aimed to conduct a systematic review and meta-analysis to investigate the impact of statin therapy on EAT.
Methods
Search strategy
The current study was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [20]. PubMed, Web of Science, Scopus, ClinicalTrials.gov, and Google Scholar databases were used for the following search terms in titles and abstracts: (“statin” or “HMG-CoA reductase inhibitor” OR “lipid lowering agents” OR “Atorvastatin” OR “Pravastatin” OR “Fluvastatin” OR “Simvastatin” OR “Rosuvastatin” OR “Lovastatin” OR “Pitavastatin”) AND (epicardial adipose tissue OR epicardial fat). The wild-card term ‘‘*’’ was used to improve the sensitivity of the search strategy. The search was performed from inception to September 5th, 2023.
Study selection
Clinical studies were included if they met the following inclusion criteria: (i) trials investigating the effect of statin on EAT, (ii) presentation of enough information at baseline and following therapy in patients providing the net change values. Also, exclusion criteria were: (i) no sufficient information at baseline or follow-up, (ii) case studies or reviews, (iii) non-English studies.
Data extraction
The authors conducted a data extraction process by removing duplicate studies. Two independent and blinded evaluators screened the titles and abstracts of the remaining publications for eligibility. We obtained and reviewed complete reports of studies that were potentially eligible, with any discrepancies being settled through discussion with a third author until a consensus was reached. The eligible studies were then reviewed, and data such as the first author information, publication date, design of the study, type of statin used, dosage of statin, treatment duration, patient information, and outcome were abstracted.
Quality assessment
To determine the quality of the studies in this meta-analysis, the Cochrane guidelines were followed. The risk of bias was evaluated based on various factors such as selection bias, detection bias, performance bias, attrition bias, reporting bias, and other sources of bias. Each of the included studies was assessed to be either high, low or unclear in terms of these biases.
Quantitative data synthesis
The Comprehensive Meta-Analysis (CMA) V4 software [21] was utilized to conduct a meta-analysis, where standardized mean differences (SMDs) were calculated based on sample size, means, and standard deviations from each group. To account for study design, treatment duration, and population characteristics, a random-effects model (using the Der Simonian-Laird method) and the generic inverse variance weighting method were used [22]. The effect sizes were shown as SMD and 95% confidence interval (CI).
Results
Study selection process
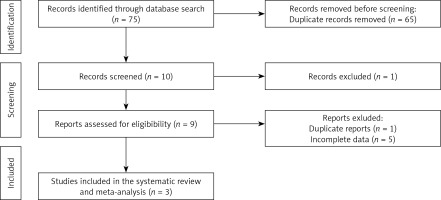
Briefly, 75 papers were initially obtained following the search through the aforementioned databases; 65 of these were excluded upon reviewing the titles and abstracts. Next, 9 full-text articles were checked and six were discarded for duplicate reports (n = 1) and incomplete data (n = 5). Thus, three studies were included in this meta-analysis (Table I). The study selection process is presented in Figure 1.
Table I
Demographic characteristics of the included studies
| Author | Study design | Target population | Treatment duration | N | Measurement of EAT | Study groups |
|---|---|---|---|---|---|---|
| Nerlekar et al. 2020 [31] | Randomized controlled trial | Coronary atherosclerosis | 4.3 years | 54 36 | CT coronary angiography | Statin treatment Control |
| Raggi et al. 2019 [16] | Randomized, double-blind, controlled | Postmenopausal women | 52 weeks | 194 226 | CT imaging | Atorvastatin 80 mg/day Pravastatin 40 mg/day |
| Soucek et al. 2015 [32] | Randomized, double-blind, placebo-controlled | Atrial fibrillation | 12 weeks | 38 41 | CT imaging | Atorvastatin 80 mg/day Placebo |
Effects of statins on EAT in RCTs
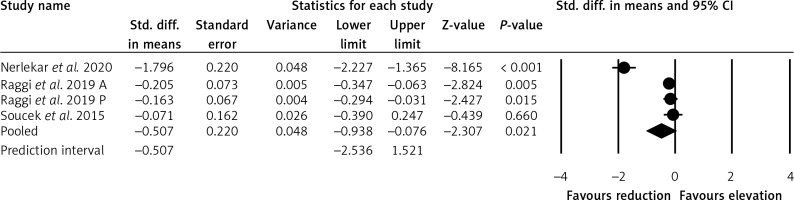
The meta-analysis of three trials including 512 patients suggested a significant reduction in EAT after statin treatment (SMD = –0.507, 95% CI: –2.536, 1.521, p = 0.021) (Figure 2).
Quality assessment
Risk of bias in this meta-analysis was evaluated based on the Cochrane instructions [23] (Table II).
Table II
Quality of bias assessment of the included studies according to the Cochrane guidelines
| Study | Sequence generation | Allocation concealment | Blinding of participants, personnel and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Nerlekar et al. 2020 [31] | L | U | H | L | L | U |
| Raggi et al. 2019 [16] | U | U | U | L | L | U |
| Soucek et al. 2015 [32] | L | L | U | L | L | L |
Discussion
Based on the findings of our meta-analysis, it is evident that the use of statins as a treatment option might lead to a significant reduction in EAT. It is important to note that excessive EAT has been linked to an increased risk of cardiometabolic complications, as well as fatal and non-fatal coronary events [15, 16]. Therefore, reducing the harmful consequences associated with excessive EAT could potentially aid in CVD prevention. While previous research has demonstrated the positive impact of lifestyle modifications such as diet and exercise, as well as bariatric surgery, on reducing EAT [24, 25], there are currently no pharmacological interventions specifically designed for EAT reduction. To date, lipid-lowering drugs such as statins have been tested as potential treatments for reducing EAT. Intensive statin therapy has shown a beneficial effect on reducing EAT, and this effect seems to be dose-dependent [13]. Importantly, this reduction in EAT is not solely due to the lipid-lowering properties of statins [16]. Parisi et al. suggested that statins may have anti-inflammatory properties that directly correlate with the thickness of EAT and can reduce inflammation in cultured EAT adipocytes [17]. Additionally, statins may affect EAT thickness through the modulation of peroxisome proliferator-activated receptors (PPARs) [26]. However, our meta-analysis revealed a significant but small impact of statins on reducing EAT. Our research findings may support the concept that atherosclerosis is not solely an issue within the blood vessels, but a disease that may also affect perivascular tissues [27, 28]. Evidence suggests that lipids may “invade” the inner layer of the vasa vasorum [27–29]. Furthermore, recent studies indicated that intensive lipid-lowering treatment can reduce the presence of vasa vasorum around the carotid artery in humans [13]. Recently, there has been growing interest in considering EAT as a potential target for therapeutic interventions aimed at modifying adipose tissue [30]. It is crucial to thoroughly investigate the mechanisms behind the abnormal accumulation of EAT in order to develop innovative and effective therapies that specifically target this tissue. We acknowledge several potential limitations in our study. Firstly, the diagnosis of EAT was determined using various imaging techniques in the analyzed publications. However, we used SMD to combine the results from various diagnostic approaches. Secondly, there was a high degree of statistical heterogeneity among the included studies. Nevertheless, the results remained consistent when sensitivity analysis was performed. Lastly, the follow-up duration varied across the analyzed studies.
In conclusion, statins may have additional potential benefits beyond lowering plasma LDL-C levels. Here, we showed how these drugs can potentially exert a therapeutic effect by mitigating EAT. However, further research is necessary to establish standardized methods for quantifying and identifying normal EAT values, as well as to enhance our knowledge of the cellular and molecular processes that lead to EAT dysregulation. Finally, it remains to be clarified whether the reducing effect on EAT is a function of the intensity of statin therapy.