Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
PEDIATRICS / SYSTEMATIC REVIEW/META-ANALYSIS
Efficacy and safety of rituximab in children with steroid-dependent or frequently relapsing nephrotic syndrome: a meta-analysis of randomized controlled trials
1
Shanghai Children’s Medical Center (SCMC) affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Submission date: 2022-11-30
Final revision date: 2023-02-09
Acceptance date: 2023-02-26
Online publication date: 2023-02-27
Corresponding author
Li Zhao
Shanghai Children’s Medical Center (SCMC) affiliated to Shanghai Jiao Tong University School of Medicine Shanghai, China
Shanghai Children’s Medical Center (SCMC) affiliated to Shanghai Jiao Tong University School of Medicine Shanghai, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
To explore the efficacy and safety of rituximab (RTX) in children with steroid-dependent nephrotic syndrome (SDNS) or frequently relapsing nephrotic syndrome (FRNS) through meta-analysis.
Material and methods:
Meta-analysis searches were performed before November 30th, 2021, using the PubMed, Embase, Web of Science, and Cochrane Library databases to collect randomized controlled trials (RCTs). FRNS or SDNS children younger than 18 years of age were included. The RTX group was treated with RTX combined with conventional therapy, whereas the control group was given conventional therapy. Review Manager 5.3 and STATA 15.0 were used to perform the statistical analyses.
Results:
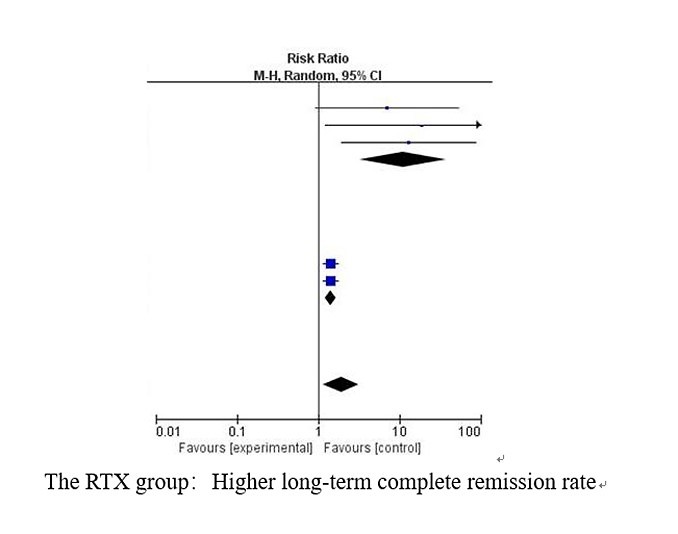
Of 1450 screened articles, a total of eight studies eligible for inclusion involving 476 patients were included. As compared to the control group (RR = 1.91, 95% CI: 1.16, 3.14, p < 0.05), RTX did not show significant improvement in the short term (RR = 2.48, 95% CI: 0.85, 7.25, p = 0.10). However, the RTX group achieved a higher short-term complete remission rate when two studies with heterogeneity were excluded (RR = 2.17, 95% CI: 1.65, 2.84, p < 0.001). Proteinuria levels were reduced more effectively in the RTX group (MD = –1.84, 95% CI: –2.42, –1.26, p < 0.001). The RTX group and the control group had no significant differences in adverse events.
Conclusions:
RTX can be considered as an effective and safe treatment option for children with SDNS or FRNS. However, it is necessary to conduct further studies via RCTs to evaluate the persistent long-term effects.
To explore the efficacy and safety of rituximab (RTX) in children with steroid-dependent nephrotic syndrome (SDNS) or frequently relapsing nephrotic syndrome (FRNS) through meta-analysis.
Material and methods:
Meta-analysis searches were performed before November 30th, 2021, using the PubMed, Embase, Web of Science, and Cochrane Library databases to collect randomized controlled trials (RCTs). FRNS or SDNS children younger than 18 years of age were included. The RTX group was treated with RTX combined with conventional therapy, whereas the control group was given conventional therapy. Review Manager 5.3 and STATA 15.0 were used to perform the statistical analyses.
Results:
Of 1450 screened articles, a total of eight studies eligible for inclusion involving 476 patients were included. As compared to the control group (RR = 1.91, 95% CI: 1.16, 3.14, p < 0.05), RTX did not show significant improvement in the short term (RR = 2.48, 95% CI: 0.85, 7.25, p = 0.10). However, the RTX group achieved a higher short-term complete remission rate when two studies with heterogeneity were excluded (RR = 2.17, 95% CI: 1.65, 2.84, p < 0.001). Proteinuria levels were reduced more effectively in the RTX group (MD = –1.84, 95% CI: –2.42, –1.26, p < 0.001). The RTX group and the control group had no significant differences in adverse events.
Conclusions:
RTX can be considered as an effective and safe treatment option for children with SDNS or FRNS. However, it is necessary to conduct further studies via RCTs to evaluate the persistent long-term effects.
REFERENCES (39)
1.
Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr 2016; 4: 39.
2.
Churg J, Habib R, White RH. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet 1970; 760: 1299-302.
3.
Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol 2017; 12: 332-45.
4.
Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child 1982; 57: 544-8.
5.
Tarshish P, Tobin JN, Bernstein J, Edelmann CJ. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997; 8: 769-76.
6.
Lombel RM, Gipson DS, Hodson EM; Kidney Disease: Improving Global Outcomes. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 2013; 28: 415-26.
7.
Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines: application to the individual patient. Kidney Int 2012; 82: 840-56.
8.
Sinha A, Bagga A. Rituximab therapy in nephrotic syndrome: implications for patients’ management. Nat Rev Nephrol 2013; 9: 154-69.
9.
MacIsaac J, Siddiqui R, Jamula E, et al. Systematic review of rituximab for autoimmune diseases: a potential alternative to intravenous immune globulin. Transfusion 2018; 58: 2729-35.
10.
Sun L, Xu H, Shen Q, et al. Efficacy of rituximab therapy in children with refractory nephrotic syndrome: a prospective observational study in Shanghai. World J Pediatr 2014; 10: 59-63.
11.
Kamei K, Ishikura K, Sako M, Ito S, Nozu K, Iijima K. Rituximab therapy for refractory steroid-resistant nephrotic syndrome in children. Pediatr Nephrol 2020; 35: 17-24.
12.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100.
13.
Ahn YH, Kim SH, Han KH, et al. Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: a multicenter open-label trial in Korea. Medicine 2018; 97: e13157.
14.
Basu B, Sander A, Roy B, et al. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. Jama Pediatr 2018; 172: 757-64.
15.
Boumediene A, Vachin P, Sendeyo K, et al. NEPHRUTIX: a randomized, double-blind, placebo vs rituximab-controlled trial assessing T-cell subset changes in minimal change nephrotic syndrome. J Autoimmun 2018; 88: 91-102.
16.
Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014; 384: 1273-81.
17.
Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011; 6: 1308-15.
18.
Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 2015; 26: 2259-66.
19.
Ravani P, Lugani F, Pisani I, et al. Rituximab for very low dose steroid-dependent nephrotic syndrome in children: a randomized controlled study. Pediatr Nephrol 2020; 35: 1437-44.
20.
Solomon N, Lalayiannis AD. Rituximab is more effective than tacrolimus in steroid-dependent nephrotic syndrome. Arch Dis Child Educ Pract Ed 2019; 104: 279-80.
21.
Gao X, Wang Y, Xu Z, Deng H, Yang H, Zhong F. Systematic review and meta-analysis of rituximab for steroid-dependent or frequently relapsing nephrotic syndrome in children. Front Pediatr 2021; 9: 626323.
22.
Liu S, Gui C, Lu Z, Li H, Fu Z, Deng Y. The efficacy and safety of rituximab for childhood steroid-dependent nephrotic syndrome: a systematic review and meta-analysis. Front Pediatr 2021; 9: 728010.
23.
Chang D, Gong M, Liu C, Zhang Q, Hu Z, Li Z. Efficacy and safety of rituximab for childhood refractory nephrotic syndrome: a meta-analysis of randomized controlled trials. Med Clin 2021; 157: 418-26.
24.
Magnasco A, Ravani P, Edefonti A, et al. Rituximab in children with resistant idiopathic nephrotic syndrome J Am Soc Nephrol 2012; 23: 1117-24.
25.
Sinha A, Bagga A, Gulati A, Hari P. Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr Nephrol 2012; 27: 235-41.
26.
Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 2010; 5: 2207-12.
27.
Hofstra JM, Deegens JK, Wetzels JF. Rituximab: effective treatment for severe steroid-dependent minimal change nephrotic syndrome? Nephrol Dial Transplant 2007; 22: 2100-2.
28.
Sergeeva T, Ananin P, Dmitrienko S, Komarova O, Semikina E. Rituximab treatment for refractory steroid-dependent nephrotic syndrome (SDNS) in children: a single-center study. Pediatr Nephrol 2017; 32: 1773.
29.
Wang L, Zhu J, Xia M, Hua R, Deng F. Comparison of rituximab, cyclophosphamide, and tacrolimus as first steroid-sparing agents for complicated relapsing/steroid-dependent nephrotic syndrome in children: an evaluation of the health-related quality of life. Arch Med Sci 2022; 18: 275-8.
30.
Kim JH, Park E, Hyun HS, et al. Long-term repeated rituximab treatment for childhood steroid-dependent nephrotic syndrome. Kidney Res Clin Pract 2017; 36: 257-63.
31.
Fujinaga S, Hirano D, Nishizaki N, et al. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 2010; 25: 539-44.
32.
Kemper MJ, Gellermann J, Habbig S, et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 2012; 27: 1910-5.
33.
Subun C, Suwannahitatorn P, Webb H, Tullus K. Rituximab in childhood steroid-sensitive nephrotic syndrome: are multiple subsequent courses safe and effective? Arch Dis Child 2021; 106: 815-8.
34.
Webb H, Jaureguiberry G, Dufek S, Tullus K, Bockenhauer D. Cyclophosphamide and rituximab in frequently relapsing/steroid-dependent nephrotic syndrome. Pediatr Nephrol 2016; 31: 589-94.
35.
Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open 2018; 1: e184169.
36.
Labrosse R, Barmettler S, Derfalvi B, et al. Rituximab-induced hypogammaglobulinemia and infection risk in pediatric patients. J Allergy Clin Immunol 2021; 148: 523-32.e8.
37.
Nakamura M, Kanda S, Yoshioka Y, et al. Rituximab-induced serum sickness in a 6-year-old boy with steroid-dependent nephrotic syndrome. CEN Case Rep 2020; 9: 173-6.
38.
Bertrand Q, Mignot S, Kwon T, et al. Anti-rituximab antibodies in pediatric steroid-dependent nephrotic syndrome. Pediatr Nephrol 2022; 37: 357-65.
39.
Fujinaga S, Nishino T, Endo S, Umeda C, Watanabe Y, Nakagawa M. Unfavorable impact of anti-rituximab antibodies on clinical outcomes in children with complicated steroid-dependent nephrotic syndrome. Pediatr Nephrol 2020; 35: 2003-8.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.