Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / CLINICAL RESEARCH
Efficacy and safety of lipegfilgrastim for prophylaxis of chemotherapy-induced neutropenia in breast cancer patients in Poland
1
Department of Oncology, Jagiellonian University Medical College, Krakow, Poland
2
Department of Uro-Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
3
Teva Pharmaceuticals Polska Sp. z o.o, Poland
Submission date: 2020-11-01
Final revision date: 2021-07-29
Acceptance date: 2021-07-30
Online publication date: 2021-08-13
Corresponding author
Jakub Kucharz
Department of Uro-Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Poland
Department of Uro-Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Poland
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Lipegfilgrastim is a long-acting glycopegylated granulocyte-colony stimulating factor (G-CSF) used to prevent chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN). The aim of the current study was to obtain data on the drug efficacy and safety in real-world clinical practice.
Material and methods:
This is an exploratory analysis of Polish breast cancer patients participating in a pan-European study of lipegfilgrastim in primary and secondary prophylaxis for patients receiving cytotoxic chemotherapy (Lonquex ObsErvational Cohort Study, LEOS). Patients were followed since the start of neutropenia prophylaxis until 6 to 8 weeks after the last dose of the lipegfilgrastim . The efficacy measures were chemotherapy dose reductions, omissions, delays and the proportion of the planned cumulative dose actually delivered.
Results:
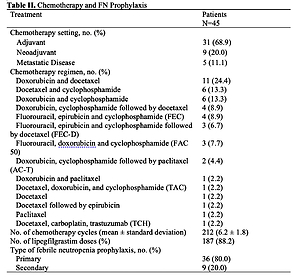
A total of 45 mostly high risk of FN patients were included in the analysis. Overall, 31 of 212 chemotherapy cycles (14.6%) were delayed in 19 patients (42.2%). Cumulative dose of chemotherapy was reduced in 1.4% of the cycles in 4.4% of the patients. The mean percentage of cumulative dose planned actually administrated was 99.95% across all cycles. Only one patient had FN. There were 15 episodes of neutropenia in 3 patients (6.7%), A total of 69 adverse events were reported, which 65% were drug-related. The most common were musculoskeletal pain (17.8%) and myalgia (11.1%) Four adverse events were serious and two of them were related to lipegfilgrastim.
Conclusions:
Lipegfilgrastim proved to be effective and well-tolerated for CIN prophylaxis in patients with breast cancer receiving myelosuppressive chemotherapy in a real-life setting.
Lipegfilgrastim is a long-acting glycopegylated granulocyte-colony stimulating factor (G-CSF) used to prevent chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN). The aim of the current study was to obtain data on the drug efficacy and safety in real-world clinical practice.
Material and methods:
This is an exploratory analysis of Polish breast cancer patients participating in a pan-European study of lipegfilgrastim in primary and secondary prophylaxis for patients receiving cytotoxic chemotherapy (Lonquex ObsErvational Cohort Study, LEOS). Patients were followed since the start of neutropenia prophylaxis until 6 to 8 weeks after the last dose of the lipegfilgrastim . The efficacy measures were chemotherapy dose reductions, omissions, delays and the proportion of the planned cumulative dose actually delivered.
Results:
A total of 45 mostly high risk of FN patients were included in the analysis. Overall, 31 of 212 chemotherapy cycles (14.6%) were delayed in 19 patients (42.2%). Cumulative dose of chemotherapy was reduced in 1.4% of the cycles in 4.4% of the patients. The mean percentage of cumulative dose planned actually administrated was 99.95% across all cycles. Only one patient had FN. There were 15 episodes of neutropenia in 3 patients (6.7%), A total of 69 adverse events were reported, which 65% were drug-related. The most common were musculoskeletal pain (17.8%) and myalgia (11.1%) Four adverse events were serious and two of them were related to lipegfilgrastim.
Conclusions:
Lipegfilgrastim proved to be effective and well-tolerated for CIN prophylaxis in patients with breast cancer receiving myelosuppressive chemotherapy in a real-life setting.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.