Emergency primary percutaneous coronary intervention (PPCI) is the preferred treatment strategy for patients with acute myocardial infarction (AMI) accompanied by heavy thrombotic load [1, 2]. Active anticoagulation and antiplatelet therapy during the PPCI perioperative period can increase PPCI’s success rate and reduce complications such as in-stent thrombosis, but it also increases the risk of bleeding. Regarding the selection of anticoagulant drugs during the PPCI perioperative period, there is no uniform standard either in China or worldwide. The problem of choosing anticoagulant drugs in clinical practice urgently needs to be solved using real-world evidence-based research.
When emergency invasive treatment strategies are adopted, the guidelines recommend using bivalirudin anticoagulation during the operation [3, 4]. Compared with traditional heparin drugs, fondaparinux has a longer action time and better efficacy and safety [5]. Several trials have shown that the risk of bleeding associated with fondaparinux therapy is lower than that associated with enoxaparin therapy [6, 7]. However, the enrolled patients in the above studies were mainly treated with thrombolytic therapy and elective percutaneous coronary intervention (PCI). By contrast, patients undergoing PPCI tend to have a heavy thrombotic load, and postoperative anticoagulant therapy strategies have not yet been agreed.
This study compared the PPCI perioperative application of bivalirudin bridging fondaparinux or enoxaparin and evaluated which treatment strategy is more effective for patients with AMI.
Methods
Trial design
The study is a registry-based, prospective, natural, and selective interventional trial that compares fondaparinux with enoxaparin in patients undergoing PPCI, using real-world data. The subjects were divided into an enoxaparin group and a fondaparinux group according to their actual clinical diagnosis and treatment. All patients were treated with bivalirudin during PPCI and were immediately given enoxaparin 40–60 mg/day or fondaparinux 2.5 mg/day after drug withdrawal, respectively. The patients were followed up for 180 days after PPCI. This study has been registered as a clinical trial with number ChiCTR1900023824 and was approved by the ethics committee of the Shanxi Cardiovascular Hospital.
Patients
Patients were eligible to be assigned to the study group if they were aged ≥ 18 years old, met the diagnostic criteria for AMI issued by the cardiovascular branch of the Chinese Medical Association and were patients undergoing PPCI treatment. In addition, it was necessary that the patient or their legal representative was informed of the nature of the study, understood the protocol, was able to ensure compliance and signed the informed consent form.
Case exclusion criteria were as follows: intraoperative IRA autolysis was found (forward TIMI blood flow ≥ 2); patients who were allergic to or with contraindications to anticoagulant and antiplatelet drugs; patients with cardiogenic shock, malignant ventricular arrhythmia, mechanical complications (such as ventricular septal rupture, papillary muscle rupture, acute mitral regurgitation, etc.); patients after coronary artery bypass graft (CABG); patients with clear indications of anticoagulation after PPCI (e.g., atrial fibrillation, left ventricular thrombosis, aortic balloon counterpulsation, pulmonary embolism, mechanical valve). Patients were also excluded if they had long-term anticoagulant indications; any bleeding tendency, severe blood disease, intracranial parenchymal aneurysm, arteriovenous malformation, suspected aortic dissection; a history of ischemic stroke, transient ischemic attack, or intracranial hemorrhage within the last six months; or a history of gastrointestinal and urinary bleeding within the last 2 weeks. Additional exclusion criteria were severe anemia and immune diseases, and/or patients undergoing hormone therapy; life expectancy with an active infection or concomitant disease such as tumor < 12 months; and coagulopathy, or moderate or severe hepatic or renal dysfunction (creatinine clearance rate < 60 ml/min/1.73 m2).
Outcome
Efficacy was evaluated by the incidence of major adverse cardiovascular and cerebrovascular events (MACCE), including death from all causes, myocardial infarction (MI), severe arrhythmia, heart failure (HF), stent thrombosis, stroke, and shock. Safety was evaluated by the incidence of bleeding. The definition and classification of bleeding in this study were based on the bleeding classification criteria developed by the Bleeding Academic Research Consortium (BARC) in 2011.
Statistical analysis
Continuous variables that followed a symmetric distribution are presented as the mean ± standard deviation (SD), and the differences between groups were analyzed using two-sample t-tests. Asymmetrically distributed continuous variables are expressed as medians with interquartile ranges, and the differences were analyzed by Wilcoxon rank-sum tests. The analyses for the categorical data expressed as percentages were performed by Pearson χ2 or Fisher exact tests. Because baseline characteristics differed between the treatment groups, additional adjustments were performed. Propensity score matching (PSM) was performed using the proximity method according to 1 : 1 to adjust the baseline disequilibrium factors (age, height, weight, sex, smoking history, hemoglobin, red blood cells) between the two groups; the matching tolerance was 0.02. After PSM, a χ2 test was used to compare the incidence of outcome events between the two groups. The hazard ratio (for fondaparinux vs. enoxaparin) and two-sided 95% confidence interval were calculated using a Cox proportional-hazards model, with the treatment group as the only covariate. Two-tailed p-values of less than 0.05 were considered to indicate statistical significance. SPSS 26.0 software was used for the data analysis.
Results
Baseline characteristics
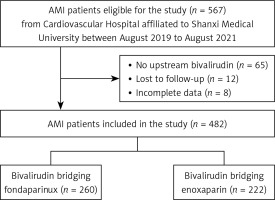
A total of 482 patients with AMI, who were attending the Shanxi Cardiovascular Hospital from August 2019 to August 2021, provided the data for this study. Patient enrolment and the flow of study participants are displayed in Figure 1. There were 222 patients in the enoxaparin group and 260 in the fondaparinux group. Patients treated with fondaparinux were on average 6.5 years older than those treated with enoxaparin, were shorter, and weighed less. Compared with the enoxaparin group, the proportion of men (76.2% vs. 91.0%) and patients smoking (64.2% vs. 75.2%) was smaller in the fondaparinux group. Regarding blood tests, patients in the fondaparinux group had lower red blood cell and hemoglobin levels. In terms of patients’ procedural characteristics, both groups were balanced. To eliminate selection bias, PSM was performed. After the match, 171 pairs of patients were screened, with the baseline and procedural characteristics being balanced between the two treatment groups (Tables I and II).
Table I
Patients’ baseline characteristics
Table II
Patients’ procedural characteristics
Clinical outcomes
Efficacy
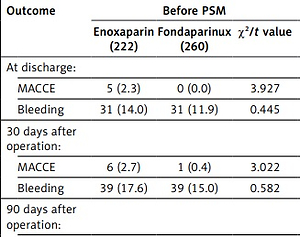
Compared with the enoxaparin group, the fondaparinux group had lower rates of MACCE (0.0% vs. 0.5%, p = 0.006) at discharge (Table III). With the extension of follow-up time, the difference in MACCE incidence between the fondaparinux and enoxaparin groups gradually decreased. By the end of the follow-up, 7.7% of patients had experienced a MACCE in the fondaparinux group, compared with 9.0% of those in the enoxaparin group (HR = 0.840, 95% CI: 0.452–1.561, p = 0.581). After PSM, there was a trend toward a lower rate of MACCE at 180 days after PPCI (9.9% vs. 4.1%; hazard ratio, HR = 0.402, 95% CI: 0.167–0.970, p = 0.042).
Table III
Major adverse clinical events in PPCI patients during follow-up time
Before matching, fondaparinux was slightly superior to enoxaparin in reducing the rate of death at the full follow-up time, but the difference was statistically insignificant significant. After matching, fondaparinux showed a significant advantage in reducing the death rate at 90 days after PPCI (1.2% vs. 5.3%, p = 0.032) and 180 days after PPCI (2.9% vs. 8.8%, p = 0.021). Among the dead patients, the incidence of recurrent myocardial infarction was higher in both groups, accounting for nearly half of all causes of death. MACCE other than death was less frequent (Table IV).
Table IV
Death and cardiovascular and cerebrovascular events at 180 days of follow-up
Safety
All patients had type 1 bleeding, and no major bleeding occurred. Overall, the fondaparinux group had a slight advantage over the enoxaparin group in terms of less bleeding over the entire follow-up period, but the difference was not statistically significant. After matching, the risk of bleeding in the fondaparinux group was significantly lower than that in the enoxaparin group at discharge (2.9% vs. 15.2%, p < 0.001) and at 30 days (5.8% vs. 19.3%, p < 0.001), 90 days (5.8% vs. 21.2%, p < 0.001), and 180 days (7.0% vs. 21.1%, p < 0.001) after PPCI (Table III).
Discussion
Currently, PPCI is the most effective treatment to reduce the mortality rate of AMI. Perioperative bleeding complications of PCI reduce patient satisfaction, delay discharge, and increase costs; furthermore, they increase the risk of death, myocardial infarction, and stroke within one year [8–10]. Hence, there is an urgent need to explore the perioperative anticoagulation strategy of PPCI.
ATOLL study was the first clinical study on the efficacy and safety of enoxaparin and unfractionated heparin (UFH) in STEMI patients undergoing PPCI. The results show that enoxaparin significantly reduced combined ischemic events such as death, infarction, and emergency revascularization, compared with UFH [11]. In the subgroup of OASIS-6 undergoing PPCI, subcutaneous injection of fondaparinux eight days after operation did not yield a clear benefit [7]. Few studies have investigated perioperative anticoagulation strategies for AMI patients undergoing PPCI, and the results are inconsistent. In addition, most studies of this type have been concentrated in Europe and America, and there are few studies on Asian races. Therefore, it is of practical and clinical significance to explore the safety and effectiveness of bivalirudin bridging fondaparinux or enoxaparin in perioperative PPCI. In general, our study found that the anticoagulation strategy of bivalirudin bridging fondaparinux seems to be superior to that of bivalirudin bridging enoxaparin in patients with AMI who are undergoing PPCI.
Bundhun et al. confirmed that fondaparinux is an ideal anticoagulant [12]. Two large-scale international clinical trials, OASIS-5 and 6, confirmed that compared with UFH or low-molecular-weight heparin (LMWH), fondaparinux had a similar anticoagulant effect on acute coronary syndrome (ACS) and significantly reduced the incidence of severe bleeding, with a significant clinical net benefit. Nevertheless, it was also found that catheter thrombosis increased significantly in patients treated with fondaparinux alone [13]. Abundant clinical trial evidence shows that bivalirudin has significant advantages in anticoagulation during PCI [14, 15]. In 2009, the US PCI guidelines recommended that bivalirudin be used in STEMI patients (Class I recommendation) and STEMI patients with a high risk of bleeding during direct PCI (Class IIa recommendation). The 2010 European Guidelines for myocardial revascularization also recommended that bivalirudin be the first choice in PCI for patients with ACS (Class I recommendation). Recently, a study from China showed that routine continuation of full-dose bivalirudin infusion for 2–4 h (mean 3 h) post-PCI is superior to UFH monotherapy in reducing bleeding and ischemic events at 30 days among patients with STEMI undergoing PPCI [16]. Bivalirudin has become a commonly used anticoagulant in emergency and elective PCI [17]. Compared with heparin or low molecular weight heparin, bivalirudin is more suitable for individuals at high risk of bleeding who require anticoagulant therapy because of its relatively low bleeding side effects.
In conclusion, the anticoagulation strategy of bivalirudin bridging fondaparinux seems to provide a favorable net clinical benefit in reducing patient mortality and bleeding risk. However, the data in this research are obtained from a single-center and non-randomized study, which may imply some selection bias. Multi-center randomized controlled trials are still needed to verify the results in the future. In addition, a longer follow-up period is needed to further confirm the efficacy and safety of bivalirudin bridging fondaparinux.