Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL PHARMACOLOGY / SYSTEMATIC REVIEW/META-ANALYSIS
Efficacy and harms associated with β-blockers for cardiotoxicity in cancer patients undergoing chemotherapy: a systematic review and meta-analysis
1
University of Connecticut School of Pharmacy, Storrs, United States
2
Oxford PharmaGenesis, Inc., Newtown, United States
3
Unidad de Revisiones Sistemáticas y Meta-análisis (URSIGET), Vicerrectorado de Investigación, Universidad San Ignacio de Loyola, Peru
Submission date: 2024-02-04
Final revision date: 2024-05-17
Acceptance date: 2024-05-30
Online publication date: 2024-06-13
Corresponding author
Adrian V. Hernandez
University of Connecticut School of Pharmacy Storrs, CT 06269, US Unidad de Revisiones Sistemáticas y Meta-análisis (URSIGET) Vicerrectorado de Investigación Universidad San Ignacio de Loyola, Lima 15024, Peru
University of Connecticut School of Pharmacy Storrs, CT 06269, US Unidad de Revisiones Sistemáticas y Meta-análisis (URSIGET) Vicerrectorado de Investigación Universidad San Ignacio de Loyola, Lima 15024, Peru
KEYWORDS
TOPICS
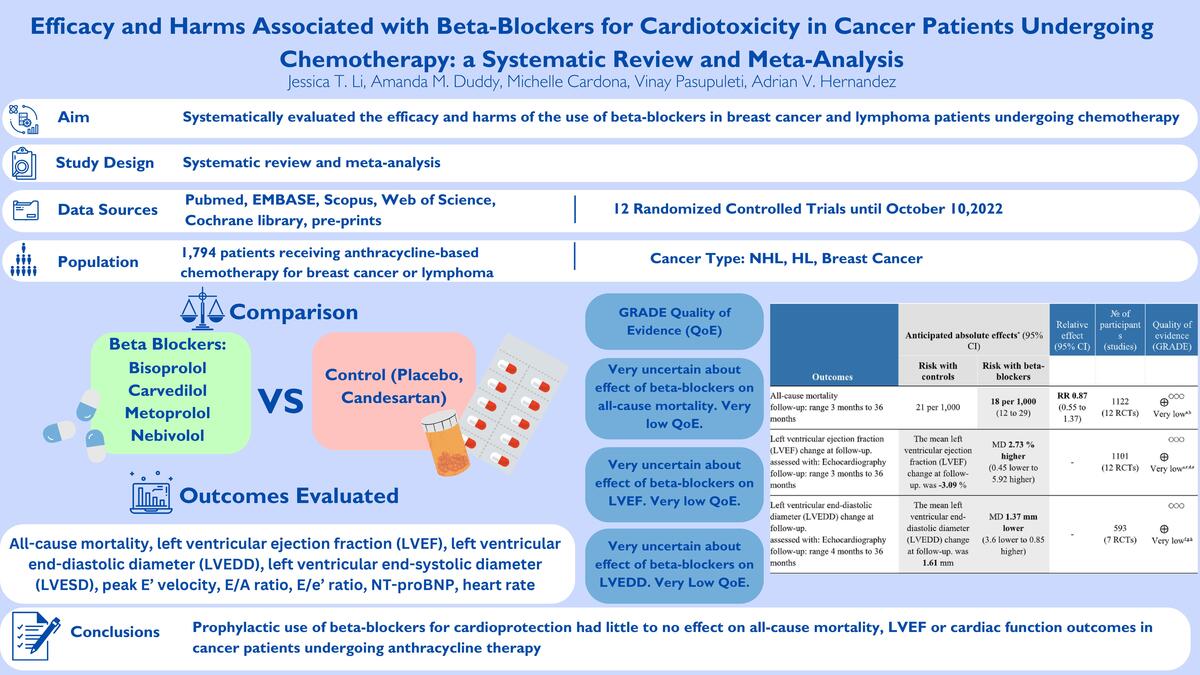
ABSTRACT
Introduction:
In patients with breast cancer and lymphoma, anthracyclines are associated with early and late dose-related cardiotoxicity. We systematically evaluated the efficacy and harms of the use of β-blockers in breast cancer and lymphoma patients undergoing chemotherapy.
Material and methods:
We searched five engines, and pre-prints until October 10, 2022, for randomized controlled trials (RCTs) evaluating β-blockers for anthracycline-associated cardiotoxicity in breast cancer and lymphoma patients. Primary outcomes were all-cause mortality, left ventricular ejection fraction (LVEF), left ventricular end-diastolic and end-systolic diameter (LVEDD, LVESD), peak E' velocity, E/A ratio, E/e' ratio, and NT-pro BNP levels. The secondary outcome was heart rate. Inverse variance random effect meta-analyses were performed, and we used GRADE methods to assess quality of evidence (QoE).
Results:
Twelve RCTs were selected (n = 1,794). Seven RCTs evaluated carvedilol. Mean ages were 39 to 52 years; 88.5% were women; 79.4% had breast cancer, and 11.5% lymphoma. The evidence was very uncertain about the effect of β-blockers on all-cause mortality (RR = 0.87, 95% CI: 0.55 to 1.37, 12 RCTs, I2 = 0%, very low QoE), LVEF (MD = 2.73%, 95% CI: –0.45% to 5.92%, 12 RCTs, I2 = 93%, very low QoE), and heart rate (MD = –9.14 bpm, 95% CI: –15.02 to –3.26, two RCTs, I2 = 87%, very low QoE) vs. controls. β-blockers likely reduced NT-pro BNP levels slightly (MD = –15.35 pg/ml, 95% CI: –22.39 to –8.31, two RCTs, I2 = 0%, moderate QoE). There were no effects on other outcomes, all with very low QoE.
Conclusions:
Prophylactic use of β-blockers for cardioprotection had little to no effect on all-cause mortality, LVEF or cardiac function outcomes in cancer patients undergoing anthracycline therapy.
In patients with breast cancer and lymphoma, anthracyclines are associated with early and late dose-related cardiotoxicity. We systematically evaluated the efficacy and harms of the use of β-blockers in breast cancer and lymphoma patients undergoing chemotherapy.
Material and methods:
We searched five engines, and pre-prints until October 10, 2022, for randomized controlled trials (RCTs) evaluating β-blockers for anthracycline-associated cardiotoxicity in breast cancer and lymphoma patients. Primary outcomes were all-cause mortality, left ventricular ejection fraction (LVEF), left ventricular end-diastolic and end-systolic diameter (LVEDD, LVESD), peak E' velocity, E/A ratio, E/e' ratio, and NT-pro BNP levels. The secondary outcome was heart rate. Inverse variance random effect meta-analyses were performed, and we used GRADE methods to assess quality of evidence (QoE).
Results:
Twelve RCTs were selected (n = 1,794). Seven RCTs evaluated carvedilol. Mean ages were 39 to 52 years; 88.5% were women; 79.4% had breast cancer, and 11.5% lymphoma. The evidence was very uncertain about the effect of β-blockers on all-cause mortality (RR = 0.87, 95% CI: 0.55 to 1.37, 12 RCTs, I2 = 0%, very low QoE), LVEF (MD = 2.73%, 95% CI: –0.45% to 5.92%, 12 RCTs, I2 = 93%, very low QoE), and heart rate (MD = –9.14 bpm, 95% CI: –15.02 to –3.26, two RCTs, I2 = 87%, very low QoE) vs. controls. β-blockers likely reduced NT-pro BNP levels slightly (MD = –15.35 pg/ml, 95% CI: –22.39 to –8.31, two RCTs, I2 = 0%, moderate QoE). There were no effects on other outcomes, all with very low QoE.
Conclusions:
Prophylactic use of β-blockers for cardioprotection had little to no effect on all-cause mortality, LVEF or cardiac function outcomes in cancer patients undergoing anthracycline therapy.
REFERENCES (45)
1.
McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther 2017; 31: 63-75.
2.
Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014; 64: 938-45.
3.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768-801.
4.
Nathan PC, Amir E, Abdel-Qadir H. Cardiac outcomes in survivors of pediatric and adult cancers. Can J Cardiol 2016; 32: 871-80.
5.
Gerodias FR Jr, Tan MK, De Guzman A, et al. Anthracycline-induced cardiotoxicity in breast cancer patients: a five-year retrospective study in 10 centers. Cardiol Res 2022; 13: 380-92.
6.
Armenian SH, Mertens L, Slorach C, et al. Prevalence of anthracycline-related cardiac dysfunction in long-term survivors of adult-onset lymphoma. Cancer 2018; 124: 850-7.
7.
Bikiewicz A, Banach M, von Haehling S, Maciejewski M, Bielecka-Dabrowa A. Adjuvant breast cancer treatments cardiotoxicity and modern methods of detection and prevention of cardiac complications. ESC Heart Fail 2021; 8: 2397-418.
8.
Naderi Y, Khosraviani S, Nasiri S, et al. Cardioprotective effects of minocycline against doxorubicin-induced cardiotoxicity. Biomed Pharmacother 2023; 158: 114055.
9.
Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017; 35: 893-911.
10.
Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout treatment: ESMO consensus recommendations. Ann Oncol 2020; 31: 171-90.
11.
Liu C, Chen H, Guo S, et al. Anti-breast cancer-induced cardiomyopathy: mechanisms and future directions. Biomed Pharmacother 2023; 166: 115373.
12.
Lewinter C, Holm Nielsen T, Edfors LR, et al. A systematic review and meta-analysis of beta-blockers and renin-angiotensin system inhibitors for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer. Eur Heart J 2022; 43: 2562-9.
13.
Kheiri B, Abdalla A, Osman M, et al. Meta-analysis of carvedilol for the prevention of anthracycline-induced cardiotoxicity. Am J Cardiol 2018; 122: 1959-64.
14.
Ma Y, Bai F, Qin F, et al. Beta-blockers for the primary prevention of anthracycline-induced cardiotoxicity: a meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol 2019; 20: 18.
15.
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160.
16.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898.
17.
Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401-6.
18.
Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016; 7: 55-79.
19.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22: 2693-710.
20.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539-58.
21.
Abuosa AM, Elshiekh AH, Qureshi K, et al. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J 2018; 70: S96-S100.
22.
Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY Trial. J Am Coll Cardiol 2018; 71: 2281-90.
23.
Ayub-Ferreira S, Avila M, Brandão S, et al. Carvedilol for prevention of chemotherapy-induced cardiotoxicity: final results of the prospective, randomized, double-blind, placebo controlled CECCY trial. J Am Coll Cardiol 2020; 75: 658.
24.
Tashakori Beheshti A, Mostafavi Toroghi H, Hosseini G, Zarifian A, Homaei Shandiz F, Fazlinezhad A. Carvedilol administration can prevent doxorubicin-induced cardiotoxicity: a double-blind randomized trial. Cardiology 2016; 134: 47-53.
25.
Cochera F, Dinca D, Bordejevic DA, et al. Nebivolol effect on doxorubicin-induced cardiotoxicity in breast cancer. Cancer Manag Res 2018; 10: 2071-81.
26.
Georgakopoulos P, Roussou P, Matsakas E, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol 2010; 85: 894-6.
27.
Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016; 37: 1671-80.
28.
Gulati G, Heck SL, Røsjø H, et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) Study. J Am Heart Assoc 2017; 6: e006513.
29.
Heck SL, Gulati G, Hoffmann P, et al. Effect of candesartan and metoprolol on myocardial tissue composition during anthracycline treatment: the PRADA trial. Eur Heart J Cardiovasc Imaging 2018; 19: 544-52.
30.
Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006; 48: 2258-62.
31.
Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol 2013; 167: 2306-10.
32.
Lee M, Chung WB, Lee JE, et al. Candesartan and carvedilol for primary prevention of subclinical cardiotoxicity in breast cancer patients without a cardiovascular risk treated with doxorubicin. Cancer Med 2021; 10: 3964-73.
33.
Livi L, Barletta G, Martella F, et al. Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol 2021; 7: 1544-9.
34.
Meattini I, Giuseppe B, Becherini C, et al. Abstract PD5-02: Cardioprotective strategy for non-metastatic breast cancer patients receiving an anthracycline-based chemotherapy: subgroup analysis focused on impact of postoperative breast radiation therapy of the preplanned interim analysis of the phase 3 SAFE trial (NCT2236806). Cancer Res 2022; 82: PD5-02.
35.
Nabati M, Janbabai G, Baghyari S, Esmaili K, Yazdani J. Cardioprotective effects of carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol 2017; 69: 279-85.
36.
Salehi R, Zamani B, Esfehani A, Ghafari S, Abasnezhad M, Goldust M. Protective effect of carvedilol in cardiomyopathy caused by anthracyclines in patients suffering from breast cancer and lymphoma. Am Heart Hosp J 2011; 9: 95-8.
37.
Xu L, Long Y, Tang X, Zhang N. Cardioprotective effects and duration of beta blocker therapy in anthracycline-treated patients: a systematic review and meta-analysis. Cardiovasc Toxicol 2020; 20: 11-9.
38.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145: e895-1032.
39.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599-726.
40.
Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013; 61: 2355-62.
41.
Jhorawat R, Kumari S, Varma SC, et al. Preventive role of carvedilol in adriamycin-induced cardiomyopathy. Indian J Med Res 2016; 144: 725-9.
42.
Liu L, Liu ZZ, Liu YY, et al. Preventive effect of low-dose carvedilol combined with candesartan on the cardiotoxicity of anthracycline drugs in the adjuvant chemotherapy of breast cancer. Zhonghua Zhong Liu Za Zhi 2013; 35: 936-40.
43.
Elitok A, Oz F, Cizgici AY, et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six-month follow-up. Cardiol J 2014; 21: 509-15.
44.
He D, Hu J, Li Y, Zeng X. Preventive use of beta-blockers for anthracycline-induced cardiotoxicity: a network meta-analysis. Front Cardiovasc Med 2022; 9: 968534.
45.
Macedo AVS, Hajjar LA, Lyon AR, et al. Efficacy of dexrazoxane in preventing anthracycline cardiotoxicity in breast cancer. JACC CardioOncol 2019; 1: 68-79.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.