Introduction
Acute kidney injury (AKI) can be defined as sudden impairment (within hours to days) of kidney function [1–3]. It is established to be an increasingly common complication in hospitalized patients. In patients, undergoing surgery perioperative AKI significantly increases their morbidity, e.g. after cardiac surgery it affects even up to 40% of patients in intensive care units (ICU) [4, 5]. In critically ill AKI mortality is very high, reaching rates of 50–80% [5, 6]. Postoperative AKI is of multifactorial origin, and besides patients’ preoperative susceptibility, many different pathways can affect renal function simultaneously [3, 7]. It seems that impaired perfusion with the inflammation and oxidative stress play a key role, while also hemodynamic instability in general, ischemia-reperfusion injury, microembolization, metabolic factors, various toxins, neurohormonal, as well as renal epithelial and endothelial activation can contribute [8–10]. The potential of deleterious effects of peri- and postoperative AKI on the patients’ outcomes [11, 12] creates an overall imperative to identify any kind of intervention, which could prevent its occurrence or shorten its postoperative course.
Statins are the most widely prescribed cholesterol-lowering drugs, being proved highly effective in both primary and secondary prevention of atherosclerosis-related cardiovascular events in different vascular territories [13–15]. The majority of data on this come from high-quality randomized, double-blind, placebo-controlled trials (RCT) in patients with coronary artery disease [16]. The efficiency of statins is directly related to their lipid-lowering effects, mainly their ability to lower low-density lipoprotein cholesterol (LDL-C) levels. Patients with concomitant cardiovascular disease (CVD) and chronic kidney disease (CKD), which frequently co-exists, are at a particularly high risk of deleterious CV events [17, 18]. In terms of lipid-lowering therapy, they should be treated early and intensively to minimize their very high risk and possibly, progression of chronic kidney disease; statins are the first line of therapy, while also ezetimibe can be safely prescribed in combination [17, 18].
In addition, there is plenty of evidence that the beneficial effects of statins may not be only due to their ability to control blood lipid levels, but also to their direct vascular and tissue-protective activities such as different anti-inflammatory, antioxidant, antithrombotic, endothelium-protective, and plaque-stabilizing activities, being called pleiotropic effects [19–25]. Statin therapy was associated with renal protection in several animal models [26, 27]. It is well known that in patients with coronary artery disease (CAD), statin use preserves renal function, since their short-term administration can reduce not only myocardial but also kidney damage in patients undergoing cardiac catheterization [28, 29]. On the other hand, statin discontinuation was associated with potentially harmful effects in patients undergoing cardiovascular surgery [30]. By exerting both lipid-lowering and a variety of lipid independent effects statins may also be considered as clinically important agents to be used in critical care settings, including also the prevention and/or attenuation of the rapid renal function decline [31, 32].
During the past two decades, several studies addressed the issue of potentially beneficial use of statins in ICU treated patients, including patients with AKI, but their results are equivocal [33]. Until now, both the effects of preadmission as well as perioperative statin use were studied [34–44]. Despite that preadmission statin therapy was shown to be associated with a lower incidence of AKI among critically ill patients, it was also suggested that this effect might not be applicable for patients with the advanced stage of renal failure [45]. Renal dysfunction which occurs postoperatively is often the result of inflammatory response and endothelial dysfunction associated with ischemia-reperfusion injury [8, 46–48]. In patients who developed AKI in the postoperative period, both preadmission and pre-(peri-)operative statin usage – being compared to participants not receiving statins – was associated with a significantly better kidney function recovery, as well as with prolonged long-term survival independent of change in kidney function in the postoperative period [41]. On the other hand, there are also data with somewhat different outcomes, which do not suggest preoperative statin use for kidney protection in adults after surgery [11, 12, 49]. In summary, until now, it was suggested that for patients not taking statins preoperatively, the treatment should not be initiated with the aim of renal protection, while on the other hand due to its well-established cardiovascular benefits patients taking statins should avoid therapy discontinuation.
With the aim to further clarify the remaining open questions, we decided to perform a systematic review of the literature and a meta-analysis of reliable and valid, high-quality studies, including also the most recent available evidence.
Material and methods
Search strategy
The guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement were followed [50]. Scopus, PubMed-Medline, ProQuest, Embase and Web of Science databases were searched until 23 June 2020 using the following search terms in titles and abstracts: (“Critical care” OR “Critically ill” OR “Critical illness” OR “critical illnesses” OR “intensive care” OR sepsis OR septic OR icu OR ccu OR “Mechanical ventilation”) AND TITLE-ABS-KEY (statins OR “statin therapy” OR “statins therapy” OR statin OR “HMG CoA reductaseinhibitor” OR lovastatin OR fluvastatin OR pravastatin OR rosuvastatin OR pitavastatin) OR TITLE-ABS-KEY (atorvastatin OR simvastatin OR cerivastatin OR lipitor OR lescol OR “Lescol XL” OR mevacor OR altoprev OR pravachol OR crestor OR zocor OR livalo). The wild-card term ‘*’ was used to increase the sensitivity of the search strategy. A manual search of the reference lists of identified studies was also performed to find additional relevant studies. The search was limited to articles published in the English language.
Study selection
Original studies were included if they met the following inclusion criteria: (1) investigating the impact of statin treatment on development of stage 1 AKI or higher, AKI mortality, ICU and hospital length of stay among subjects who underwent surgeries, (2) observational retrospective, cohort, case-control and randomized control trial study design, and (3) presentation of sufficient information on AKI development or mortality. Exclusion criteria were: (1) non-clinical studies; and (2) lack of sufficient information on AKI development or mortality.
Data extraction
The following data were collected from eligible studies: (1) first author’s name; (2) year of publication; (3) study location; (4) study design; (5) number of participants in the statin and control groups; (6) odds ratio (OR) and mean of ICU/hospital stay, AKI incidence or mortality. Two authors reviewed the papers, and disagreements were resolved through discussion and consultation with a third author.
Quality assessment
The quality of studies included in this meta-analysis was assessed according the Cochrane criteria. Risk of bias was evaluated according to the Cochrane instructions [51].
Statistical analysis and summary of synthesis
Meta-analyses were performed on the extracted and evaluated epidemiological data for dichotomous outcome variables, which included factors associated with AKI, AKI-related death and mean ICU/hospital stay. The pooled mean difference and 95% confidence intervals (CI) for mean ICU/hospital length of stay and pooled OR with 95% CI for the development of AKI and AKI mortality, were calculated for statin users compared to non-users. An OR of less than 1 indicated a reduced odd of AKI in statin users or protective effect of statin against mortality outcomes, whereas an OR of more than 1 indicated detrimental effect of statin use. Forest plots were obtained to indicate the effect of the pooled estimates of OR, using either fixed or random models with the inverse variance (IV).
We assessed heterogeneity using I2 measure and the Cochran Q-statistic within or between study designs. The null hypothesis was the absence of heterogeneity; if rejected, a random model was used to calculate pooled estimates. Assessment of publication bias was evaluated by designing a funnel plot and calculating Egger’s and Begg’s (with continuity correction) tests. The null hypothesis was that there was no publication bias in the study. STATA, version 16.0 (Stata Corp, College Station, TX), was used for statistical analysis.
Results
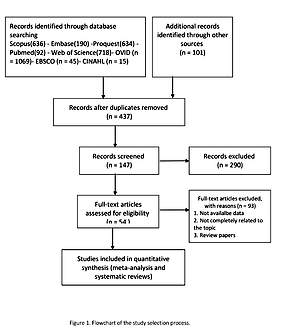
The flow diagram of the study selection process is summarized in Figure 1. The main characteristics of the studies included in the meta-analyses are shown in Table I [52–60].
Table I
Characteristics of eligible studies included in the meta-analysis
| Studies | Year of publication | Study period | Multi-/single center | Country/ region | Study design | Patients | Number of statin users | Number of statin non-users | Included endpoint | Type and dose of statin |
|---|---|---|---|---|---|---|---|---|---|---|
| Saeed [52] | 2017 | – | Single | Iran | Randomized control trial | Hospitalized in ICU | 35 | 35 | ICU LOS | Atorvastatin 40 mg daily |
| Malbouisson [53] | 2019 | – | Single | Brazil | Prospective cohort study | Hospitalized in ICU | 122 | 548 | ICU LOS HOS LOS Mortality | NA |
| Chello [54] | 2006 | 2003–2004 | Single | Italy | Randomized control trial | Hospitalized for elective surgery | 20 | 20 | ICU LOS HOS LOS | Atorvastatin 20 mg daily |
| Zheng [12] | 2016 | Single | China | Randomized control trial | Hospitalized for elective cardiac surgery | 960 | 962 | ICU LOS HOS LOS | Rosuvastatin 20 mg daily | |
| Prowle [55] | 2012 | Single | Australia | Randomized control trial | Hospitalized for elective cardiac surgery | 50 | 50 | ICU LOS HOS LOS | Atorvastatin 40 mg once daily for 4 days starting preoperatively | |
| Kor [56] | 2008 | 2004–2005 | Single | USA | Retrospective cohort study | Hospitalized in surgical ICU | 89 | 62 | ICU LOS HOS LOS | NA |
| Billings [11] | 2016 | 2009–2014 | Single | USA | Randomized control trial | Hospitalized for elective cardiac surgery | 308 | 307 | ICU LOS AKI development Mortality | NA |
| Murugan [57] | 2012 | - | Multi | USA | Prospective cohort study | Hospitalized with community-acquired pneumonia | 176 | 455 | HOS LOS | NA |
| Billings [58] | 2010 | - | Single | USA | Retrospective analysis of RCT | Hospitalized for elective cardiac surgery | 80 | 244 | HOS LOS AKI development | NA |
| Hsu [59] | 2018 | 2010-2013 | Multi | USA | Randomized control trial | Hospitalized in ICU | 254 | 257 | AKI development | Rosuvastatin 40 mg at the time of enrolment followed by 20 mg daily until the third day after intensive care unit discharge, study day 28, hospital discharge, or death |
| Molnar [42] | 2011 | 1995-2008 | Multi | Canada | Retrospective cohort | Hospitalized for elective surgery | 67941 | 145406 | AKI Development Mortality | 55% were on a low potency statin and 37% were on a high potency statin |
| Welten [35] | 2008 | 1995-2006 | Single | Netherlands | Cohort | Hospitalized for elective surgery | 515 | 1429 | AKI Development | NA |
| Layton [60] | 2013 | 2000-2010 | Multi | USA | Cohort | Hospitalized for cardiac surgery | 3085 | 13992 | AKI Development | 26.0% initiated a higher-potency statin and 74 % first used a low-potency statin |
Use of statins in patients diagnosed with AKI and the length of stay in ICU
Seven studies were eligible for the analysis of the effect of statin use on ICU length of stay in patients diagnosed with AKI. The analysis was conducted by using a fixed inverse variance model due to heterogeneity of variance (I2 = 0.0%, p = 0.630) (Figures 2 A, B). There was no publication bias, since the estimated bias coefficient for the Egger’s test was –0.655 with a p-value of 0.244. The Begg’s test with continuity correction gave a p-value of 0.274 (Figure 2 C). The mean difference was observed and it was not significant (MD (95% CI), –0.02 (–0.06, 0.02); p = 0.321) (Figure 2 A).
Use of statins in patients diagnosed with AKI and the length of stay in the hospital
Seven eligible studies were included in the analysis of the effect of statins on the hospital length of stay in AKI patients. The test for heterogeneity was statistically significant (I2 = 77.6%, p < 0.001), so we concluded that the fixed-effect model is not consistent with the data. Analysis was performed by using a random model (Figures 3 A, B). The Egger’s test with the estimated bias coefficient of –2.583772 and p-value = 0.061 provided evidence for the presence of small-study effects. However, Begg’s test with continuity correction (p = 0.274) indicated no publication bias (Figure 3 C). The mean difference was significant (MD (95% CI), –0.49 (–0.91, –0.07); p = 0.020) (Figure 3 A).
Use of statins in development of AKI
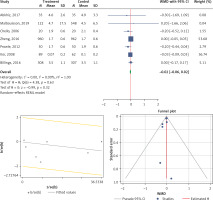
Six studies were eligible to be included in the analysis of the effect of statin use on the development of AKI. The overall heterogeneity was very high (I2 = 93.9%, p < 0.001) (Figure 4 A). A random model was applied for obtaining the estimates (Figure 4 B). There was no publication bias at the level of 1% according to the Egger’s test with estimated bias coefficient of –4.76 and p-value of 0.144 along with Begg’s test with continuity correction (p = 0.129) (Figure 4 C). Our results showed that pooling effect sizes resulted in no significant association between statin use and development of AKI (OR (95% CI), 0.92 (0.63, 1.33); p = 0.650) (Figure 4 A). Subgroup analysis showed that neither preoperative nor postoperative statin therapy was associated with the development of AKI, and the overall incidence was comparable between the two subgroups (Figure 4 D).
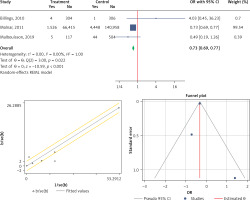
Statins effect on mortality outcome
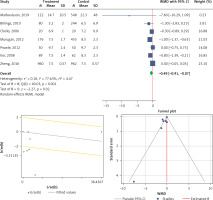
Three eligible studies were performed to analyze the effects of the statins on mortality in patients with AKI. The overall heterogeneity was I2 = 0% (p = 0.223) (Figure 5 A). Therefore, the use of a fixed model for analysis was confirmed (Figure 5 B). Both Egger’s test and Begg’s test revealed no publication bias with bias coefficient of 1.31 and p-value of 0.366 and bias with continuity correction (p = 0.999) (Figure 5 C). Our results showed a significant reduction of mortality with statin therapy (OR (95% CI), 0.73 (0.69, 0.77); p < 0.001) (Figure 5 A).
Discussion
The main finding of our meta-analysis is that statin treatment can improve the overall survival in critically ill surgical patients, who suffer from AKI and need care in the ICU. We also found that statins in general do not contribute to the AKI development, and do not affect significantly the patients’ ICU length of stay while they might reduce the overall hospital length of stay. Similarly, pre- or post-operative use of statins does not significantly affect the risk of AKI development.
In critically ill patients, the effects of statin therapy on the development of AKI, its course (including the ICU and overall hospital length of stay and the need of renal replacement therapy (RRT)), and the influence on specific and overall mortality is not unequivocal, despite in general that statin use is associated with a beneficial effect during long-term postoperative follow-up. Until now, some studies suggested a clear benefit, while in others either no benefit or even potential harm was demonstrated, which could be related to significant differences in patient populations, study designs, as well as the type and dose of the statin used [61–67].
Our main result, which is that statin use is associated with the significant overall reduction in mortality, is mainly driven by the results of the retrospective observational study within a large cohort of over 200,000 surgical patients, in which nearly one third were taking statins for more than 90 days before surgery, and approximately 2% suffered from AKI [42]. Statin use – with the similar share of patients using high- or low-potency statins – was associated with reduced risks of AKI, need of acute dialysis, and mortality (with ORs of 0.84, 0.83, and 0.79, respectively). Consistently, an observational study in patients undergoing major vascular surgery demonstrated improved long-term survival (OR = 0.60) in statin users, who were also shown much better complete kidney function recovery in case it was deteriorated [35]. In a prospective cohort study of patients consecutively admitted to the ICU, a significantly lower requirement for renal replacement therapy (RRT) and mortality was found (OR = 0.41) among approximately one fifth of patients who were taking statins prior to hospital admission [53]. Similarly, in patients treated for community-acquired pneumonia, although statins did not diminish the AKI development, their use was associated with a lower long-term follow-up risk of death [57].
Our results showed no significant association between the statin use in general and the development of AKI in our study, what is in accordance with previously published meta-analyses [39, 63]. Similarly, investigation on a large cohort from insurance billing data reported an overall neutral effect of statin use (as a class) on the development of AKI within the first year of the treatment initiation [60]. However, therapy with statins was shown moderately to reduce the rate of eGFR decline and proteinuria [68].
The exact mechanisms of both short- and long-term statin related, specific nephroprotective beneficial effects in critically ill patients who developed AKI are not known. Many pathophysiological processes follow AKI, including generation of reactive oxygen species, reduced antioxidant protection and less nitric oxide bioavailability, what can result in injury, dysfunction, as well as the death of the kidney cells. In addition, renal inflammation is implicated involving activation and diapedesis of leukocytes from the circulation, as well as increased cytokine/adhesion molecules expression. Both lower HDL-cholesterol levels and hypertriglyceridemia (characterizing the so-called metabolic syndrome of critically ill) are markers of worse prognosis in critical care patients, in whom lipoproteins can exert direct anti-inflammatory actions. It was suggested that HDL and LDL particles could represent important regulators of human response to endotoxemia, as well as that serum triglycerides frequently increase in systemic inflammatory reactions [69–72]. Thus, in critically ill patients statins might improve AKI through their so-called pleiotropic (LDL-cholesterol independent) effects with direct tissue-protective effects on the renal vasculature and tubular cells, and a systemic anti-inflammatory effect [46, 53, 73–75].
Preoperative statin treatment was proven effective (showing 22% relative risk reduction of AKI) within a large observational study of 17,000 patients who underwent cardiac surgery [76], and confirmed with a meta-analysis by which a 33% reduction in the risk of RRT was demonstrated in patients who received preoperative statin therapy before undergoing coronary artery bypass graft surgery [77]. In contrast, investigators from a similar observational study of 11,000 patients found no effect [37], and also a meta-analysis of five trials that reported AKI after cardiac surgery did not indicate any effect of statin therapy [78]. The overall similar incidence of AKI among both preoperative statin users (with a probable increased risk of atherosclerotic disease and inflammation), and statin non-users could possibly be related to the potential source of residual confounding by indication for statin use, so it could be that a nephroprotective effect of preoperative statin use could be missed.
Our analysis failed to prove any influence on the ICU length of stay, but indicated shortening of overall hospital length of stay. The subgroup analysis showed that neither preoperative nor postoperative statin use was associated with the odds of the development of AKI. It was shown before that both among chronic statin users and statin-naïve cardiac surgery patients, early postoperative statin use is associated with a lower incidence of AKI [38]. In patients undergoing coronary catheterization, statin bolus enhances the protective effect of chronic statin use on myocardium [79]. Also, statin-naïve coronary catheterization patients were protected by short-term perioperative statin treatment [80]. On this basis, it can be assumed that statin effects on oxidative products generation and renal endothelium could probably affect AKI more than statin reduction of inflammation. The response of the lipid profile to statin administration is usually slow, while on the other hand, at least in animal models, evidence of reduced tissue damage has been noted with the administration of statins as close as 24 h before the planned experimental injury [81]. Hence, in most of the trials the early postoperative statin use was defined as statin treatment within the first postoperative day.
It was reported from an observational study that the early postoperative statin administration after cardiac surgery was associated with a lower incidence of AKI among both chronic statin users and statin-naïve patients (OR = 0.32) [58]. Similarly, in patients undergoing major vascular surgery who developed AKI in the postoperative period, it was found that statin use was associated with a two-fold increase in kidney function recovery when compared to participants not receiving statins [35]. This is because in patients undergoing cardiovascular surgery, statin intervention within the first postoperative day may have a greater effect on the development of AKI than preoperative statin use, since AKI developed in this setting results from sustained injury throughout the continuum of surgery and the early postoperative period. Experimentally, it was found that within 6–18 h, statin treatment improves endothelial function and reduces the generation of reactive oxygen species (ROS) [82, 83]. Subsequently, statin use reduces endothelium-leukocyte interactions, platelet reactivity, and systemic concentrations of interleukin-6, interleukin-8, tumour necrosis factor-α, and circulating leukocyte number and activity in coronary surgery patients [19, 54]. Since early postoperative statin use was associated with less AKI, but preoperative statin use was not, statin effects on ROS generation and renal endothelium may affect AKI more than statin alteration of inflammation.
Interestingly, the reports from the observational studies are somehow in contrast with the results of a double-blind, placebo-controlled RCT among patients undergoing cardiac surgery [11]. Investigators used a differential regime for either the group of statin-naïve patients (given either one high dose, 80 mg of atorvastatin a day before surgery, followed by 40 mg on the day of the procedure as well as during the consecutive days, or matching placebo; n = 199), and for the patients already taking statins prior to the inclusion into the study (who continued with the statin from the pre-enrollment phase until the day of surgery, when they were randomly assigned to receive 80 mg on the morning of the surgery and 40 mg of atorvastatin on the morning after, while from day 2 after they continued taking the pre-trial prescribed statin, or matching placebo; n = 416). Within the entire study population, as well as in patients taking statins before the inclusion, administration of atorvastatin did not influence the development of AKI, while in statin-naïve patients the incidence of AKI was significantly higher (RR = 1.61). The authors concluded that the initiation of statin therapy to prevent AKI following cardiac surgery could not be supported.
Within the majority of interventional, in general relatively small trials where statins were applied shortly in front of the surgical procedure, atorvastatin or rosuvastatin were used, and there is a common lack of the same kind of data on some less potent statins (e.g. simvastatin, pravastatin, lovastatin) [11, 12, 52, 54, 55]. In patients scheduled for elective cardiac surgery, rosuvastatin was not beneficial but associated with a significant absolute excess in postoperative AKI [12]. Although the adverse effects on renal function may relate to the specific patients’ ethnic group or to the use of rosuvastatin, a class effect of statin therapy in patients undergoing cardiac surgery cannot be ruled out [75, 84]. Rosuvastatin also did not prevent either de novo AKI or worsening of AKI in those with preexisting mild disease in critically ill patients with acute respiratory distress syndrome (ARDS) and sepsis [59]. Previously, using rosuvastatin as the reference, atorvastatin and simvastatin had superior efficacy in preventing mortality in patients with sepsis [85].
Some authors also discussed the issue of statin withdrawal, usually defined as discontinuation of preoperative statin treatment prior to surgery until at least the second postoperative day, as another potentially important aspect of the influence of pre- and postoperative stop and start of statins on kidney functioning. While preoperative statin use was not shown to affect the risk, statin withdrawal nearly doubled the incidence of AKI [58]. In addition, chronic statin users who had their therapy reinitiated in the early postoperative period were found to have significantly better indicators, e.g. improvements of AKI, the need for RRT, as well as the overall hospital length of stay [30, 34]. In another study, the continuing statin group showed a lower elevation of more accurate biomarkers for diagnosing AKI in its earlier stages (urine interleukin-18, urine neutrophil gelatinase-associated lipocalin, urine kidney injury molecule-1, and plasma neutrophil gelatinase-associated lipocalin) when compared to patients who interrupted statin assumption. On this basis, it can be suggested not only that statins should not be interrupted in patients chronically taking them, but also that they may actually perform as anti-inflammatory and endothelial sparing agents in AKI, as well as that the use of new biomarkers could provide us with higher sensitivity to identify susceptible patients.
Several limitations related to our analysis are worth mentioning before the conclusion. Since there is a general lack of apparent standardization of care for the perioperative management of patients undergoing major surgery, the data from selected trials are subject of the potentially significant variation. The study populations included are very diverse, and the potential influence of mild to moderate baseline CKD is generally excluded from the study reports. Large, prospective, randomized and well-controlled clinical trials in less heterogeneous study populations are needed to answer the questions on the length and dose of the treatment, as well as on the most appropriate time point for possible discontinuation of statins before surgery and re-initiation. These could be particularly useful if they combine “classic” AKI criteria with more sensitive biomarkers aiming for better selection of patients and investigation of particular drugs in a standardized fashion.
In conclusion, our current meta-analysis confirmed an important beneficial association between the statin use and reduction of the overall mortality of critically ill patients diagnosed with AKI. At the same time statins were not shown to influence the overall development of AKI or affect the ICU length of stay but reduced the overall hospital length of stay. Despite the use of statins does not significantly influence the development of AKI in general, subgroup analysis demonstrated that for both pre- and postoperative statin use there was no association with AKI risk. Nevertheless, based on this and other recent high-quality evidence, patients already receiving statins chronically should not discontinue this therapy for being useless for AKI prevention or potentially harmful.