Introduction
Sepsis, defined as severe organ dysfunction caused by a dysregulated host response to infection, has a high incidence and high mortality rate worldwide [1–4]. Several conventional indicators, such as procalcitonin (PCT) and C-reactive protein (CRP), may reflect the severity of infection [5, 6]. The Simplified Acute Physiology Score (SAPS) and the Sequential Organ Failure Assessment (SOFA) score play important roles in evaluating the severity and prognosis of critical illnesses [7, 8]. Notably, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) are novel predictors of the prognosis of sepsis [9–13].
Similarly, the lymphocyte-to-monocyte ratio (LMR), which can be easily derived from the complete blood count, is closely correlated with the severity and prognosis of several clinical diseases. A decreased LMR is reportedly predictive of a poor prognosis of acute ischaemic stroke (AIS) [14, 15]. Also, the inflammatory response participates in the pathophysiology of AIS [16]. However, no previous study has explored the relationship between LMR and the prognosis of sepsis in a large population.
The SAPS and SOFA scores allow assessment of the prognosis of septic patients; however, the subitems concerning white blood cell (WBC) components, which change significantly during an inflammatory response, are incorporated into neither. Therefore, we evaluated the relationship of the LMR with the short- and long-term outcomes of sepsis in critically ill patients at the time of admission to the intensive care unit (ICU).
Material and methods
Patients
Data for this study were extracted from the Medical Information Mart for Intensive Care (MIMIC) clinical database (v. 1.4), which contains comprehensive deidentified clinical data of patients admitted to the Beth Israel Deaconess Medical Center in Boston, Massachusetts [17]. MIMIC III contains data on more than 58,000 distinct adult and neonate admissions to critical care units from 2001 to 2012. The Institutional Review Board of the Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology approved the use of the MIMIC III database for authorised users who have completed the required training (Wei Zhou; ID: 25222342). The requirement for individual patient consent was waived for this study because it did not impact clinical care and all health information was deidentified [17].
Patients diagnosed with sepsis according to the criteria of Angus et al. were enrolled [18]. We selected patients with severe sepsis, ICD-9 codes for bacterial or fungal infection, and a diagnosis of acute organ dysfunction. Only patients aged 18 years or older with lymphocyte and monocyte counts obtained at ICU admission were included. The exclusion criteria were as follows: (1) date of death unclear, (2) definite haematological malignancies, (3) repeat ICU admissions, and (4) incomplete patient data for multivariate analysis.
Data collection
The data extracted from the MIMIC III database comprised gender, age, results of laboratory tests, comorbidities, special treatments during ICU stay, and ICU length of stay (LOS). The SAPS and SOFA scores on admission were calculated from the database [7, 8]. The baseline laboratory data and illness scores were evaluated during the first 24 h of the ICU stay.
Target and outcome variables
We recorded the complete blood count of each patient during the first 24 h of ICU admission. The LMR was calculated as the absolute lymphocyte count to absolute monocyte count ratio. The maximum value of the LMR (LMRmax) was the target variable.
The primary end point was 28-day survival and the secondary end point was 1-year survival. Mortality was calculated based on dates of admission and death, obtained from the MIMIC III database.
Statistical analysis
Numerical variables were evaluated for a normal distribution using the Kolmogorov-Smirnov normality test. Non-normally distributed data are presented as medians with interquartile ranges. Categorical variables are presented as frequency with percentage. Differences in the non-normally distributed variables were evaluated using the Wilcoxon rank-sum test. The Pearson χ2 test and the Fisher exact test were used for analysing categorical variables. By receiver operating characteristic (ROC) curve analysis, the cut-off value of 3.0 (taking the integer of the cut-off value in two ROC curves), which was confirmed using Youden’s index, was regarded as the optimal point for distinguishing low-LMRmax and high-LMRmax groups for further statistical analysis.
We assessed the association of LMRmax with the risk of death at 28 days and 1 year by univariate logistic regression. The results are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). We generated Kaplan-Meier (K-M) curves to analyse the probability of survival and compared the results among the LMRmax groups using the log-rank test.
A Cox regression analysis was performed to identify whether LMRmax was independently associated with the prognosis of septic patients. The hazard ratio (HR) and 95% CI were calculated for the final model. After removing collinear factors, the following variables were adjusted for in the multivariable analysis: gender, age, laboratory test results (haemoglobin and lactate levels), SOFA and SAPS scores, alcohol abuse, comorbidities (congestive heart failure, cardiac arrhythmias, hypertension, chronic pulmonary, renal failure, liver disease, solid tumour, and diabetes), and LOS in the ICU.
Two-sided p-values of < 0.05 were considered indicative of statistical significance. All statistical analyses were performed using SPSS software (ver. 20.0; IBM Corp., Armonk, NY).
Results
Baseline characteristics of participants
A total of 3,087 patients were included in this study. The baseline characteristics of the low- and high-LMRmax groups are listed in Table I. The two groups were significantly different in terms of gender ratio and age. Compared to the low-LMRmax group, the high-LMRmax group had a lower WBC count, creatinine level, and SAPS score (all p < 0.01), and a longer duration of mechanical ventilation and ICU LOS (both p < 0.01). With regard to comorbidities, the high-LMRmax group exhibited lower frequencies of cardiac arrhythmias (p < 0.01), chronic pulmonary (p < 0.05), renal failure (p < 0.01) and solid tumour (p < 0.05) than the low-LMRmax group. Additionally, comparison of baseline characteristics of the study cohort vs. the missing data cohort is presented in Table II.
Table I
Baseline characteristics of the study participants
| Characteristics | Total (N = 3087) | Low-LMRmax (≤ 3) (N = 1409) | High-LMRmax (> 3) (N = 1678) |
|---|---|---|---|
| Gender (men/women) | 1664/1423 | 792/617 | 872/806a |
| Age [years], n (%): | |||
| > 20, ≤ 40 | 88 (2.9) | 20 (1.4) | 68 (4.0)b |
| > 40, ≤ 60 | 603 (19.5) | 251 (17.8) | 352 (21.0)a |
| > 60, ≤ 80 | 1322 (42.8) | 617 (43.8) | 705 (42.0) |
| > 80 | 1074 (34.8) | 521 (37.0) | 553 (33.0)a |
| Alcohol abuse, n (%) | 231 (7.5) | 108 (7.7) | 123 (7.3) |
| Lab items: | |||
| WBC [× 109/l] | 12.90 (8.60–18.30) | 14.20 (10.30–20.10) | 11.80 (7.50–16.80)b |
| Hemoglobin [g/dl] | 10.90 (9.50–12.50) | 10.80 (9.50–12.50) | 10.90 (9.50–12.50) |
| Lactate [mmol/l] | 2.20 (1.50–3.60) | 2.10 (1.40–3.50) | 2.20 (1.50–3.80) |
| Creatinine [g/dl] | 1.40 (0.90–2.40) | 1.50 (0.90–2.60) | 1.40 (0.90–2.20)b |
| SOFA score | 6 (4–9) | 6 (4–9) | 6 (4–9) |
| SAPS score | 47 (38–57) | 48 (39–58) | 46 (38–56)b |
| Mechanical ventilation (first 24 h), n (%) | 1811 (58.7) | 812 (57.6) | 999 (59.5) |
| Duration of mechanical ventilation [h] | 24.00 (0–134.67) | 18.03 (0–111.50) | 31.00 (0–158.26)b |
| Vasopressor, n (%) | 1657 (53.7) | 750 (53.2) | 907 (54.1) |
| Duration of vasopressor [h] | 3.13 (0–38.97) | 2.52 (0–38.79) | 3.43 (0–39.10) |
| Comorbidities, n (%): | |||
| Congestive heart failure | 1250 (40.5) | 578 (41.0) | 672 (40.0) |
| Cardiac arrhythmias | 1178 (38.2) | 573 (40.7) | 605 (36.1)b |
| Hypertension | 1648 (53.4) | 776 (55.1) | 872 (52.0) |
| Chronic pulmonary | 755 (24.5) | 374 (26.5) | 381 (22.7)a |
| Renal failure | 707 (22.9) | 361 (25.6) | 346 (20.6)b |
| Liver disease | 403 (13.1) | 193 (13.7) | 210 (12.5) |
| Solid tumor | 184 (6.0) | 98 (7.0) | 86 (5.1)a |
| Diabetes | 911 (29.5) | 399 (28.3) | 512 (30.5) |
| ICU LOS [days] | 4.13 (1.97–8.91) | 3.77 (1.89–7.70) | 4.53 (2.02–10.17)b |
Table II
Baseline characteristics of study cohort and missing data cohort
Association of LMRmax with 28-day and 1-year survival
The 28-day and 1-year survival rates were 47.9% and 19.9%, respectively, in the low-LMRmax group, and 60.4% and 25.9%, respectively, in the high-LMRmax group. Univariate logistic regression analyses revealed that the high-LMRmax group had higher 28-day and 1-year survival rates than the low-LMRmax group (both p < 0.001).
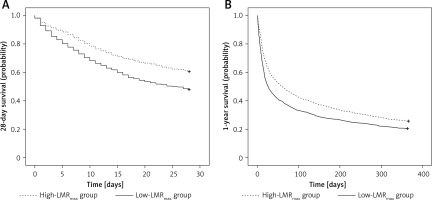
Furthermore, in the K-M analysis of 28-day and 1-year survival, patients with a high LMRmax had a more favourable prognosis than those with a low LMRmax (both p < 0.001) (Figure 1 A, B).
Figure 1
Kaplan-Meier (K-M) survival analysis for low-LMRmax and high-LMRmax group. A – 28-day survival curve; B – 1-year survival curve. The curves demonstrated that the patients with high LMRmax had higher probability of survival than those with low LMRmax at 28 days and 1 year (both p < 0.001)
LMR – lymphocyte-to-monocyte ratio.
Subgroup analysis of LMRmax
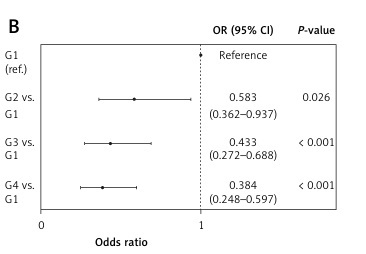
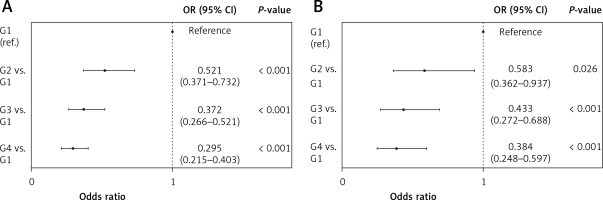
Based on the values of LMRmax, the subjects were divided into four groups: group 1 (LMRmax ≤ 1, n = 203), group 2 (LMRmax > 1 and ≤ 2, n = 572), group 3 (LMRmax > 2 and ≤ 3, n = 634), and group 4 (LMRmax > 3, n = 1,678). In univariate logistic regression analyses, there was a significant stepwise decrease in the risk of death at both 28 days and 1 year from group 1 to group 4 (Figures 2 A, B).
Figure 2
Univariate logistic regression analysis for subgroups. A – 28-day survival; B – 1-year survival. There was a statistically significant stepwise decrease in the risk of death at 28 days and 1 year from group 1 to group 4 (G1: LMRmax ≤ 1; G2: LMRmax > 1, ≤ 2; G3: LMRmax > 2, ≤ 3; G4: LMRmax > 3)
CI – confidence interval, G – group, LMR – lymphocyte-to-monocyte ratio, OR – odds ratio.
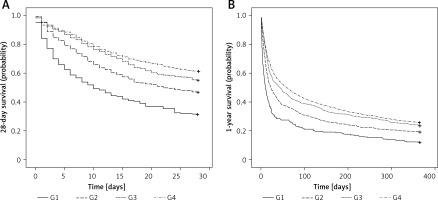
The K-M 28-day and 1-year survival curves suggested significant differences in survival probability among the four groups (both p < 0.001) (Figures 3 A, B).
Figure 3
Kaplan-Meier (K-M) survival analysis for subgroups. A – 28-day survival curve; B – 1-year survival curve. The curves demonstrated that the patients of group 4 (maximum LMRmax) had the highest probability of survival at 28 days and 1 year, and the patients of group 1 (minimum LMRmax) had the lowest probability of survival at 28 days and 1 year (both p < 0.001)
G – group, LMR – lymphocyte-to-monocyte ratio.
Association of LMRmax with survival after multivariable adjustments
The association of LMRmax with survival after multivariable adjustments was evaluated by Cox regression analysis. As presented in Table III, after adjustment for gender, age, haemoglobin and lactate levels, SOFA and SAPS scores, alcohol abuse, comorbidities, and ICU LOS, the risk of death at 28 days (group 1: reference; group 2: p = 0.001, HR = 0.709; group 3: p < 0.001, HR = 0.556; group 4: p < 0.001, HR = 0.479) and 1 year (group 1: reference; group 2: p = 0.001, HR = 0.741; group 3: p < 0.001, HR = 0.599; group 4: p < 0.001, HR = 0.580) showed significant stepwise decreases from group 1 to group 4.
Table III
Cox regression analysis for 28-day survival and 1-year survival
[i] CI – confidence interval, ICU – intensive care unit, LMR – lymphocyte-to-monocyte ratio, LOS – length of stay, HR – hazard ratio, SAPS – Simplified Acute Physiology Score, SOFA – Sequential Organ Failure Assessment. The variables for multivariable adjustments: gender, age, lab items (hemoglobin and lactate), scores of SOFA and SAPS, state of alcohol abuse and comorbidities (congestive heart failure, cardiac arrhythmias, hypertension, chronic pulmonary, renal failure, liver disease, solid tumor and diabetes), and ICU LOS.
Discussion
In this study, septic patients with a high LMRmax were more likely to survive, and had a better prognosis at 28 days and 1 year after hospital admission. The 28-day and 1-year survival rates increased significantly as the LMRmax increased. There was an independent and positive association between LMRmax and the 28-day and 1-year survival rates.
Sepsis, a leading cause of mortality worldwide, is defined as a syndrome of physiological, pathological, and biochemical abnormalities induced by infection [19–22]. The incidence of sepsis is increasing [23, 24]. Moreover, a considerable proportion of septic patients require treatment at the time of ICU admission [18]. Severe sepsis occurs when infection causes acute organ dysfunction, mediated by the inflammatory response [1, 2, 25]. Thus, it is appropriate to extract septic patients in the MIMIC III database using the criteria of Angus et al. [18].
Lymphocytes are involved in the pathophysiological inflammatory response of septic patients [26]. Sepsis-induced lymphocyte apoptosis, characterised by a decrease in quantity of lymphocytes and suppression of the immune response, is associated with a protracted course, infectious complications, and poor prognosis in septic patients [27–29]. Pro-inflammatory mediators produced by monocytes play a pivotal role in the pathogenesis of sepsis [30]. Based on their surface expression of CD14 and CD16, human monocytes can be subdivided into three subtypes: (1) classical monocytes, phagocytes with no inflammatory attributes; (2) non-classical monocytes, a subpopulation with inflammatory features; and (3) intermediate monocytes, a subpopulation with both phagocytic and inflammatory functions [31]. Therefore, it is confirmed that lymphocytes are a protective factor, while monocytes are a harmful factor in the process of inflammation.
Silva et al. reported that a lower LMR was independently associated with a higher risk of 6-month mortality after discharge among patients with acute heart failure [32]. Also, a lower LMR was independently associated with mortality in patients with hepatitis B virus-related liver cirrhosis [33], malignant haematological disorders [34, 35], and peripheral arterial diseases [36]. Therefore, the LMR, which comprises the effects of both lymphocytes and monocytes, could be predictive of the prognosis of critically ill patients. In line with previous reports, we found that a lower LMRmax was associated with increased risk of short- and long-term mortality in septic patients, suggesting that the responses of lymphocytes and monocytes to inflammation, and the interaction between these two subtypes of leukocyte, are involved in the inflammatory cascade and subsequent recovery. The LMRmax value better reflects the continuous changes that occur during ICU admission. However, no study of the LMRmax has been reported, to our knowledge.
The LMR is determined from the counts of lymphocytes and monocytes, the activation, apoptosis, necrosis, and cytokine production of which are involved in the pathogenesis of sepsis [37]. Naess et al. demonstrated that, compared to patients with fever of non-infectious causes, patients with fever due to bacterial infection had a lower LMR, whereas those with viral infection had a higher LMR; this may be useful in the diagnosis of bacterial infection and selection of antibiotics [38]. Monocyte to lymphocyte ratios in the extreme percentiles (< 9% or > 25%) are reportedly associated with active tuberculosis [39]. Moreover, Djordjevic et al. suggested that the LMR value was not predictive of the outcomes of critically ill patients with peritonitis and pancreatitis, but was predictive of the severity of bacteraemia [40]. We found that the correlation between the LMRmax and survival was independent of sepsis-related scores; thus, an inflammation-related reaction may be involved in the recovery from sepsis.
The molecular mechanism underlying the role of LMR in survival is unclear. In septic lymphocytes, decreased expression of T-bet, GATA3, and ROR-γt, which regulate Th1, Th2, and Th17 effector CD4 T cells, was observed [29, 41]. Furthermore, the mediation of microvesicular caspase-1 may induce apoptosis of lymphocytes after sepsis [42]. Also, lower HLA-DR and fractalkine receptor (CX3CR1) expression on monocytes was correlated with a poor immunosuppression outcome in patients with clinical sepsis [43, 44]. Also, the number of CD16+ monocytes, which after stimulation produce the pro-inflammatory cytokine tumour necrosis factor but not anti-inflammatory factors, was markedly increased in response to sepsis [45, 46]. Moreover, Giamarellos-Bourboulis et al. reported that early apoptosis of blood monocytes was a mechanism of protection in sepsis, which in part supports our findings [47].
This study had several limitations. First, the retrospective design of the study renders it vulnerable to selection bias, where participants were drawn from only a single centre, patients with missing data were excluded and causality could not be determined. Second, we did not compare the effect of fungal and bacterial sepsis on prognosis. Third, we merely analysed the maximum value of LMR at ICU admission without dynamic observation, which perhaps affects the association between LMR and prognosis in septic patients. Thus, further prospective studies with continuous monitoring of the LMR are warranted to validate our findings.
In conclusion, we report for the first time that a lower LMRmax value was an independent predictor of a poor prognosis in septic patients, which provides clinical evidence regarding the mechanism underlying sepsis. Therefore, as an inexpensive and readily available indicator, LMRmax may facilitate stratification of prognosis in septic patients.