Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / CLINICAL RESEARCH
ERK knockdown suppresses cell biological activities via regulation of CD59 in breast cancer
1
Jiangxi University of Traditional Chinese Medicine, Nanchang, China
2
Department of Breast Surgery, The Third Hospital of Nanchang, Jiangxi, China
3
Jiangxi Province Key Laboratory for Breast Diseases Nanchang, Jiangxi, China
Submission date: 2021-05-24
Final revision date: 2021-06-10
Acceptance date: 2021-07-02
Online publication date: 2021-08-07
Corresponding author
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The purpose of this study was to investigate the effect of extracellular signal-regulated kinase (ERK) in breast cancer and the related mechanisms.
Material and methods:
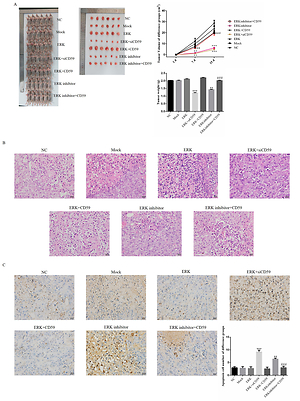
Some previous studies found that ERK was closely correlated with CD59 in cancer. In our clinical study, we evaluated ERK and CD59 protein expression in different tissue from patients by immunohistochemical (IHC) assay using MCF-7 and MDA-MB-231 cell lines which were breast cancer cell lines as target cell lines. We performed an in vitro study, evaluating cell biological activities including proliferation, apoptosis, cell cycle, invasion, adherent and migration by MTT, clone test, TUNEL assay, flow cytometry and wound healing, and measuring relative protein expression by WB assay. In an in vivo study, measuring tumor weight and volume, the apoptosis cell number was evaluated by TUNEL assay and relative protein expression by IHC assay.
Results:
Compared with adjacent normal tissue, the ERK and CD59 protein expression levels were significantly increased in breast cancer tissues (both p < 0.001). In in vitro and in vivo studies, with ERK knockdown, the cell biological activities were significantly depressed with CD59 suppression (both p < 0.001). Also the relative protein levels including CD59, PKD, P53, E-cadherin and vimentin were significantly different (each p < 0.001).
Conclusions:
ERK act as an oncology gene in breast cancer development. ERK inhibitor suppressed breast cancer biologically via regulation of CD59 in vitro and in vivo.
The purpose of this study was to investigate the effect of extracellular signal-regulated kinase (ERK) in breast cancer and the related mechanisms.
Material and methods:
Some previous studies found that ERK was closely correlated with CD59 in cancer. In our clinical study, we evaluated ERK and CD59 protein expression in different tissue from patients by immunohistochemical (IHC) assay using MCF-7 and MDA-MB-231 cell lines which were breast cancer cell lines as target cell lines. We performed an in vitro study, evaluating cell biological activities including proliferation, apoptosis, cell cycle, invasion, adherent and migration by MTT, clone test, TUNEL assay, flow cytometry and wound healing, and measuring relative protein expression by WB assay. In an in vivo study, measuring tumor weight and volume, the apoptosis cell number was evaluated by TUNEL assay and relative protein expression by IHC assay.
Results:
Compared with adjacent normal tissue, the ERK and CD59 protein expression levels were significantly increased in breast cancer tissues (both p < 0.001). In in vitro and in vivo studies, with ERK knockdown, the cell biological activities were significantly depressed with CD59 suppression (both p < 0.001). Also the relative protein levels including CD59, PKD, P53, E-cadherin and vimentin were significantly different (each p < 0.001).
Conclusions:
ERK act as an oncology gene in breast cancer development. ERK inhibitor suppressed breast cancer biologically via regulation of CD59 in vitro and in vivo.
REFERENCES (31)
1.
Burnett RM, Craven KE, Krishnamurthy P, et al. Organ-specific adaptive signaling pathway activation in metastatic breast cancer cells. Oncotarget 2015; 6: 12682-96.
2.
Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9-29.
3.
Lim E, Lin NU. Updates on the management of breast cancer brain metastases. Oncology 2014; 28: 572-8.
4.
Cho MS, Vasquez HG, Rupaimoole R, et al. Autocrine effects of tumor-derived complement. Cell Rep 2014; 6: 1085-95.
5.
Fishelson Z, Donin N, Zell S, et al. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol 2003; 40: 109-23.
6.
Goswami MT, Reka AK, Kurapati H, et al. Regulation of complement-dependent cytotoxicity by TGF--induced epithelial-mesenchymal transition. Oncogene 2016; 35: 1888-98.
7.
Mamidi S, Cinci M, Hasmann M, et al. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol 2013; 7: 580-94.
8.
Terp MG, Lund RR, Jensen ON, et al. Identification of markers associated with highly aggressive metastatic phenotypes using quantitative comparative proteomics. Cancer Genomics Proteomics 2012; 9: 265-73.
9.
Liu M, Yang YJ, Zheng H, et al. Membrane-bound complement regulatory proteins are prognostic factors of operable breast cancer treated with adjuvant trastuzumab. Oncol Rep 2014; 32: 2619-27.
10.
Simões AE, Rodrigues CM, Borralho PM. The MEK5/ERK5 signalling pathway in cancer: a promising novel therapeutic target. Drug Discov Today 2016; 21: 1654-63.
11.
Ouyang Q, Zhang L, Jiang Y, et al. The membrane complement regulatory protein CD59 promotes tumor growth and predicts poor prognosis in breast cancer. Int J Oncol 2016; 48: 2015-24.
12.
Wang Y, Hoeppner LH, Angom RS, et al. Protein kinase D up-regulates transcription of VEGF receptor-2 in endothelial cells by suppressing nuclear localization of the transcription factor AP2. J Biol Chem 2019; 294: 15759-67.
13.
Zhang Y, Wang HH, Wan X, et al. Inhibition of protein kinase D disrupts spindle formation and actin assembly during porcine oocyte maturation. Aging 2018; 10: 3736-744.
14.
Li QQ, Hsu I, Sanford T, et al. Protein kinase D inhibitor CRT0066101 suppresses bladder cancer growth in vitro and xenografts via blockade of the cell cycle at G2/M. Cell Mol Life Sci 2018; 75: 939-63.
15.
Maier D, Nagel AC, Kelp A, et al. Protein kinase D is dispensable for development and survival of Drosophila melanogaster. G3 (Bethesda) 2019; 9: 2477-87.
16.
Qin XJ, Gao ZG, Huan JL, et al. Protein kinase D1 inhibits breast cancer cell invasion via regulating matrix metalloproteinase expression. Eur J Gynaecol Oncol 2015; 36: 690-3.
17.
Bonfim-Melo A, Zanetti BF, Ferreira ÉR, et al. Trypanosoma cruzi extracellular amastigotes trigger the protein kinase D1-cortactin-actin pathway during cell invasion. Cell Microbiol 2015; 17: 1797-810.
18.
Bernhart E, Damm S, Heffeter P, et al. Silencing of protein kinase D2 induces glioma cell senescence via p53-dependent and -independent pathways. Neuro Oncol 2014; 16: 933-45.
19.
Ryvkin V, Rashel M, Gaddapara T, et al. Opposing growth regulatory roles of protein kinase D isoforms in human keratinocytes. J Biol Chem 2015; 290: 11199-208.
20.
Luo Y, Fu X, Ru R, et al. CpG oligodeoxynucleotides induces apoptosis of human bladder cancer cells via caspase-3-Bax/Bcl-2-p53 axis. Arch Med Res 2020; 51: 233-44.
21.
Alijani Ardeshir R, Rastgar S, Morakabati P, et al. Selective induced apoptosis and cell cycle arrest in MCF7 and LNCap cell lines by skin mucus from round goby (Neogobius melanostomus) and common carp (Cyprinus carpio) through P53 expression. Cytotechnology 2020; 72: 367-76.
22.
Zhu X, Luo C, Lin K, et al. Overexpression of DJ-1 enhances colorectal cancer cell proliferation through the cyclin-D1/MDM2-p53 signaling pathway. Biosci Trends 2020; 14: 83-95.
23.
Yang B, Bai H, Sa Y, et al. Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J Cancer 2020; 11: 2303-17.
24.
Moreno-Celis U, López-Martínez FJ, Cervantes-Jiménez R, et al. Tepary bean (Phaseolus acutifolius) lectins induce apoptosis and cell arrest in G0/G1 by P53(Ser46) phosphorylation in colon cancer cells. Molecules 2020; 25: 1021.
25.
Cheng CS, Chen JX, Tang J, et al. Paeonol inhibits pancreatic cancer cell migration and invasion through the inhibition of TGF-1/Smad signaling and epithelial-mesenchymal-transition. Cancer Manag Res 2020; 12: 641-51.
26.
Xu Y, Yan YC, Hu YK, et al. WWOX regulates the Elf5/Snail1 pathway to affect epithelial-mesenchymal transition of ovarian carcinoma cells in vitro. Eur Rev Med Pharmacol Sci 2020; 24: 1041-53.
27.
Arisan ED, Akar RO, Rencuzogullari O, et al. The molecular targets of diclofenac differs from ibuprofen to induce apoptosis and epithelial mesenchymal transition due to alternation on oxidative stress management p53 independently in PC3 prostate cancer cells. Prostate Int 2019; 7: 156-65.
28.
Wang X, Chen S, Shen T, et al. Trichostatin A reverses epithelial-mesenchymal transition and attenuates invasion and migration in MCF-7 breast cancer cells. Exp Ther Med 2020; 19: 1687-94.
29.
Verma S, Kang AK, Pal R. BST2 regulates interferon gamma-dependent decrease in invasion of HTR-8/SVneo cells via STAT1 and AKT signaling pathways and expression of E-cadherin. Cell Adh Migr 2020; 14: 24-41.
30.
Kim H, Shin S, Kim Y, et al. The clinicopathologic significance of extranodal tumor extension in locally advanced (pT3) colorectal adenocarcinoma and its association with the loss of E-cadherin expression. Int J Clin Exp Pathol 2019; 12: 3417-25.
31.
Xu X, Zhu H, Yang M, et al. Knockdown of TOR signaling pathway regulator suppresses cell migration and invasion in non-small cell lung cancer via the regulation of epithelial-to-mesenchymal transition. Exp Ther Med 2020; 19: 1925-32.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.