Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
Does trastuzumab-related cardiotoxicity influence
long-term outcome in patients with HER-2 positive
breast cancer? A prospective study
1
Cardiology Department, Nicolaus Copernicus Memorial Hospital, Lodz, Poland
2
Department of Cardiooncology, Medical University, Lodz
3
Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge, United Kingdom
4
Department of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge, United Kingdom
5
Department of Hypertension, Medical University of Lodz, Lodz, Poland
Submission date: 2020-04-29
Final revision date: 2020-05-24
Acceptance date: 2020-05-25
Online publication date: 2021-03-16
Corresponding author
Grzegorz Piotrowski
Cardiooncology Department, Medical University of Lodz, Lodz, Poland Department of Cardiology, M. Kopernik Specialist District Hospital, Łódź,, Pabianicka 62, 93-513, Lodz, Poland
Cardiooncology Department, Medical University of Lodz, Lodz, Poland Department of Cardiology, M. Kopernik Specialist District Hospital, Łódź,, Pabianicka 62, 93-513, Lodz, Poland
KEYWORDS
cardiotoxicitydisease-free survivaloverall survivalbreast canceradjuvant treatmentnon-metastatic breast cancerbreast cancer prognosis
TOPICS
ABSTRACT
Introduction:
Trastuzumab is a monoclonal antibody directed against the HER-2 receptor that has led, in an adjuvant setting, to higher disease-free (DFS) and overall survival (OS) in HER-2 positive breast cancer (BC) compared with chemotherapy alone. Cardiotoxicity often results in early discontinuation of trastuzumab, which may elevate the risk of cancer recurrence or mortality. Our study aimed to assess how early interruption or early permanent termination of adjuvant trastuzumab treatment influences DFS and OS of patients with HER-2 positive BC.
Material and methods:
This is a prospective observation of 253 women (55 ±10 years of age) with HER-2 positive unilateral, non-metastatic BC treated with trastuzumab in an adjuvant setting. To monitor the safety of the treatment echocardiography was performed at baseline and every 3 months up to 12 months after the end of therapy. If cardiotoxicity developed, trastuzumab was stopped early. Overall survival and DFS were assessed.
Results:
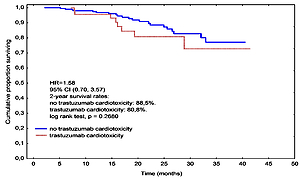
Trastuzumab-associated cardiac complications resulting in treatment discontinuation developed in 52 (20.55%) patients. Median DFS time was 21.1 months in the group with interruption compared with 25.7 months in the group with full trastuzumab treatment, being significantly shorter (HR = 2.32, 95% CI: 1.15–4.71, p = 0.0106). Two year OS in the interruption and no-interruption groups were 80.8% and 88.5%, respectively, which were not statistically significantly different (p = 0.268). In a multivariate regression analysis the cumulative dose of anthracycline (OR = 1.01, 95% CI: 1.00–1.01, p = 0.002) and LVEF at baseline (OR = 0.83, 95% CI: 0.70–0.99, p = 0.0344) were independent predictors of a cardiotoxic effect.
Conclusions:
Trastuzumab-related cardiotoxicity resulting in early treatment discontinuation negatively influences DFS, but does not seem to influence OS.
Trastuzumab is a monoclonal antibody directed against the HER-2 receptor that has led, in an adjuvant setting, to higher disease-free (DFS) and overall survival (OS) in HER-2 positive breast cancer (BC) compared with chemotherapy alone. Cardiotoxicity often results in early discontinuation of trastuzumab, which may elevate the risk of cancer recurrence or mortality. Our study aimed to assess how early interruption or early permanent termination of adjuvant trastuzumab treatment influences DFS and OS of patients with HER-2 positive BC.
Material and methods:
This is a prospective observation of 253 women (55 ±10 years of age) with HER-2 positive unilateral, non-metastatic BC treated with trastuzumab in an adjuvant setting. To monitor the safety of the treatment echocardiography was performed at baseline and every 3 months up to 12 months after the end of therapy. If cardiotoxicity developed, trastuzumab was stopped early. Overall survival and DFS were assessed.
Results:
Trastuzumab-associated cardiac complications resulting in treatment discontinuation developed in 52 (20.55%) patients. Median DFS time was 21.1 months in the group with interruption compared with 25.7 months in the group with full trastuzumab treatment, being significantly shorter (HR = 2.32, 95% CI: 1.15–4.71, p = 0.0106). Two year OS in the interruption and no-interruption groups were 80.8% and 88.5%, respectively, which were not statistically significantly different (p = 0.268). In a multivariate regression analysis the cumulative dose of anthracycline (OR = 1.01, 95% CI: 1.00–1.01, p = 0.002) and LVEF at baseline (OR = 0.83, 95% CI: 0.70–0.99, p = 0.0344) were independent predictors of a cardiotoxic effect.
Conclusions:
Trastuzumab-related cardiotoxicity resulting in early treatment discontinuation negatively influences DFS, but does not seem to influence OS.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.