Introduction
Autoimmune thyroid disease (AITD) is the most prevalent organ-specific autoimmune disease (AID) worldwide, and it consists of classical forms, autoimmune hyperthyroidism, and autoimmune thyroiditis [1]. Autoimmune hyperthyroidism, or Graves’ disease (GD), is attributed to an antibody-mediated immune response and features the generation of a thyroid-stimulating hormone (TSH) receptor (TSHR) antibody (TSHRAb) against the TSHR, which functions as a TSH agonist and binds to the TSHR, and subsequently stimulates thyrocyte hyperplasia and thyroid enlargement, and promotes thyroid-specific gene expressions and thyroid hormone production [1]. On the other hand, autoimmune thyroiditis, or Hashimoto’s thyroiditis (HT), is primarily induced by a cell-mediated immune response, and subsequently results in thyrocyte apoptosis, thyroid follicle destruction, diffuse immunocyte infiltration, and ultimately thyroid failure [2]. Despite the distinct mechanisms of the two diseases, evidence has shown that a genetic predisposition and environmental triggers are fundamental contributors to the occurrence of GD and HT [2].
Type 1 interferon (IFN) is secreted by many cell types, and is chiefly induced by viral infections or certain cellular elements [3]. Type 1 IFN comprises two main types, IFN-α and -β, which are typically produced by plasma dendritic cells and fibroblasts, respectively [4]. Dysregulation of the IFN-α signature is known to be pathogenic in several kinds of AIDs, including Sjögren’s syndrome (SS), systemic lupus erythematous (SLE), etc. [5]. Interferon α is used as an important treatment scenario for malignant diseases and viral infections in clinical settings. Moreover, IFN-α administration in chronic hepatitis C patients is well known to contribute to thyroid autoantibody formation and the occurrence of subclinical and overt AITD [6]. On the other hand, in addition to enhancing antiviral and adaptive immune reactions, IFN-β was shown to elicit a remarkable immunomodulatory effect, and is broadly used in therapy for relapsing-remitting multiple sclerosis (MS) [7]. However, the actual influences of IFN-β on thyroid function and thyroid autoimmunity remain controversial [8, 9].
B cells are critical for maintaining humoral immunity, and an imbalance of B cell functions can lead to the formation of autoantibodies and the occurrence of AIDs [10]. B-cell activating factor (BAFF), mainly released by monocytes, has a powerful role in establishing the entire B cell capacity of the body [11]. Accumulating evidence has shown that serum BAFF levels participate in AIDs, including SLE and rheumatoid arthritis [12, 13]. In our previous findings, serum BAFF levels were elevated in GD, HT, and AITD and could modify the phenotype of GD [14]. Krumbholz et al. observed that IFN-β can induce BAFF production both in vivo and in vitro, and suggested that IFN-β could be a strong BAFF stimulator [15]. However, the relationship between IFN-β and BAFF in AITD is unknown.
In the present study, we measured serum IFN-β levels in patients with GD, HT, and in healthy controls in an ethnic Chinese (i.e., Taiwanese) population and evaluated the pathogenic role of serum IFN-β levels in the occurrence and clinical features of AITD. In addition, the association of IFN-β with BAFF levels in different groups was also determined.
Material and methods
Samples
Autoimmune thyroid disease blood samples were obtained by the Division of Endocrinology, Internal Department, Shuang Ho Hospital (New Taipei City, Taiwan) from January 2013 to September 2014, and included 160 GD and 47 HT patients. Blood samples of 120 participants without AIDs were obtained by the Health Screening Center of Shuang Ho Hospital from May to August 2014. Participates were excluded if they were aged younger than 20 years, pregnant, alcoholic, or had a history of drug intoxication. The study protocol was approved by the Joint Institutional Review Board of Taipei Medical University, and all participants provided written informed consent prior to participation. Graves’ disease and HT were diagnosed according to the literature [16]. As GD with anti-thyroid peroxidase antibody (anti-TPO) could be attributed to concurrent thyroiditis in GD, we divided GD patients into two groups according to the presence or absence of anti-TPO [17, 18]. The body-mass index (BMI) was calculated as (body weight in kg)/(height in m)2.
Laboratory analyses
Serum free thyroxine (FT4) and TSH were determined by electrochemiluminescence immunoassay methods (Roche Diagnostics, USA). Serum anti-TPO and anti-thyroglobulin antibody (anti-Tg) titers were determined by particle agglutination methods (Fujirebio, Japan). A reciprocal titer of ≥ 1 : 100 was considered positive. Serum TSHRAb levels were quantified by a radioimmunoassay method (R.S.R., UK), and > 15% was considered positive [19].
Serum IFN-β and BAFF levels were measured by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Patients with AITD (a combination of the 160 patients with GD and 47 patients with HT) were stratified into low (< 4.035 pg/ml) and high IFN-β (≥ 4.035 pg/ml) groups based on the median value of the serum IFN-β level (4.035 pg/ml).
Statistical analysis
All statistical analyses were performed using SPSS software, vers. 13.0 for Windows (SPSS, Chicago, IL, USA). Thyroid-stimulating hormone and IFN-β concentrations were not normally distributed and so were logarithmically transformed. An independent t-test was used to evaluate the demographic data, thyroid function, IFN-β, and TSHRAb between two groups. The χ2 test or Fisher’s exact test was also used to assess differences in categorical data between the two groups. Pearson’s correlation was used to determine the correlations of variables. Associations of the occurrence of HT with clinical variables, including IFN-β levels, and demographic data were first evaluated by a univariate logistic regression analysis. For instance, when HT was defined as the dependent variable (0 for the control, 1 for HT), the variables of interest, i.e., age, sex, a family history (FH) of thyroid diseases, and smoking, were taken as the independent variables. Significantly discriminating factors were determined and further included in the multivariate logistic regression for analysis. All statistical tests were two-sided, and a p-value of < 0.05 was considered significant.
Results
Demographic data of the Graves’ disease, Hashimoto’s thyroiditis, autoimmune thyroid disease, and control groups
Table I shows the demographic data of different groups. The percentage of women and the number of patients with an FH were higher in the AITD group than in the control group. Hashimoto’s thyroiditis patients were older than GD patients and controls, and the ratio of women among HT patients was higher compared to those in GD patients and control subjects. Graves’ disease and HT patients had stronger FHs than did the controls. Graves’ disease and AITD patients had lower BMI values than the control group. In the GD group, 3 patients were found to simultaneously have ankylosing spondylitis. Meanwhile, 128 of 160 GD patients were under antithyroid drug medication, while 38 of 47 HT patients were receiving levothyroxine sodium replacement. Odds ratios (ORs) and 95% confidence intervals (CIs) of the demographic data are shown in Supplementary Table SI.
Table I
Demographic characteristics in the Graves’ disease (GD), Hashimoto’s thyroiditis (HT), autoimmune thyroid disease (AITD), and control groups
| Parameter | Control (n = 119) | AITD (n = 207) | GD (n = 160) | HT (n = 47) |
|---|---|---|---|---|
| Age [years] (minimum–maximum) | 43.3 ±11.1d (24–72) | 43.4 ±13.3 (20–86) | 41.7 ±12.6d (20–86) | 49.0 ±14.6a,c (26–84) |
| Women, n (%) | 74 (62.2)b,d | 156 (75.4)a | 114 (71.9)d | 41 (87.2)a,c |
| Smokers, n (%) | 17 (14.7) | 43 (20.9) | 38 (23.9)d | 5 (10.6)c |
| Family history of thyroid disease, n (%) | 4 (3.4)b,c,d | 51 (26.7)a | 44 (30.6)a,d | 7 (14.9)a,c |
| Body mass index (BMI) [kg/m2] | 24.2 ±3.6b,c | 22.7 ±3.9a | 22.5 ±4.0a | 23.1 ±3.4 |
| Other autoimmune diseases | 0 | 3(1.45) | 3 (1.88) | 0 |
Comparison of serum IFN-β levels among the different groups
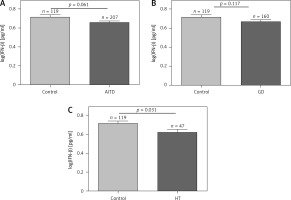
Associations of IFN-β levels with GD, HT, and AITD patients and control subjects are shown in Figure 1. There was no significant difference in IFN-β levels between AITD and controls or between GD patients and controls (Figures 1 A, B), while HT had lower IFN-β levels than the control (p = 0.031, Figure 1 C). Serum IFN-β levels did not significantly differ between the GD and HT groups (p = 0.179, data not shown).
Associations of serum IFN-β levels with thyroid function, and TSHRAb, anti-TPO, and anti-Tg levels
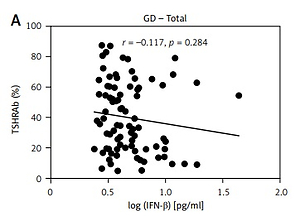
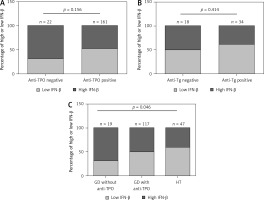
Results of thyroid function and TSHRAb levels in each group are shown in Supplementary Table SII. Patients in the GD group had higher FT4 levels than those in the HT group. In addition, GD patients had lower TSH levels compared to the controls and HT patients. There was no association of FT4 levels with IFN-β levels in all, female, or male AITD patients (Supplementary Figure S1). As shown in Figure 2, there was no significant correlation between serum IFN-β levels and TSHRAb titers at enrollment in all GD patients (r = –0.117, p = 0.284) or in women with GD (r = 0.002, p = 0.987), but a significant association was observed in men with GD (r = –0.433, p = 0.044). On the other hand, there was no significant association of serum IFN-β levels with the presence of anti-TPO or anti-Tg (Figures 3 A, B) or with anti-TPO and anti-Tg titers (data not shown). Interestingly, there was a trend of decreasing serum IFN-β levels from GD without anti-TPO, GD with anti-TPO, to HT (p = 0.046, Figure 3 C). In order to re-confirm the findings, we obtained another cutoff using a receiver operating characteristic curve. The cutoff of IFN-β levels to determine a high risk for predicting AITD was 4.328 pg/ml with a sensitivity of 60.5% and a specificity of 59.4%. Herein, we stratified AITD patients into low (< 4.328 pg/ml) and high IFN-β (≥ 4.328 pg/ml) groups based on the cutoff. Results of the associations of thyroid autoantibody with IFN-β are shown in Supplementary Figure S2, and there was still a tendency of declining IFN-β levels from GD to HT (p = 0.029, Supplementary Figure S2 C). Meanwhile, there was no significant association of serum IFN-β levels with the presence or titers of anti-TPO or anti-Tg in either women or men (data not shown). In addition, there were no significant differences in serum IFN-β levels TSHRAb, or anti-TPO titer between women and men in normal subjects, or in the AITD, GD, or HT groups (Supplementary Table SIII).
Figure 2
Association of serum thyroid-stimulating receptor antibody (TSHRAb) levels with interferon-β levels in all, female, and male patients with Graves’ disease (GD) at enrollment. P < 0.05 indicates statistical significance
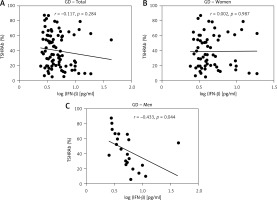
Figure 3
Associations of low or high interferon (IFN)-β levels with the presence of an antithyroid peroxidase antibody (anti-TPO, A), the presence of an antithyroglobulin antibody (anti-Tg, B) in autoimmune thyroid disease, and the association of INF-b levels with Graves’ disease (GD) patients without anti-TPO, GD patients with anti-TPO, and patients with Hashimoto’s thyroiditis (HT) (C). P < 0.05 indicates statistical significance. The low IFN-β group is defined by IFN-β < 4.035 pg/ml, while the high IFN-β group is defined by IFN-β ≥ 4.035 pg/ml
Alterations of serum IFN-β levels in different stages of GD and HT
To assess alterations of serum IFN-β levels in different stages of GD, we classified GD patients into three groups: acute GD (GD patients with new onset or recurrence prior to medication or those receiving an antithyroid drug (ATD) within 3 months after disease onset), chronic GD (GD patients receiving ATD for more than 3 months), and remission GD (GD patients who had discontinued ATD and were in a remission status according to the status in which we collected the blood samples). On the other hand, we also assessed the difference in IFN-β levels between the drug naive (LT4(–)) group and the group undergoing levothyroxine sodium replacement (LT4(+)). No significant association of serum IFN-β levels with the disease status was detected in GD or HT (Supplementary Table SIV).
Association of serum IFN-β levels with BAFF
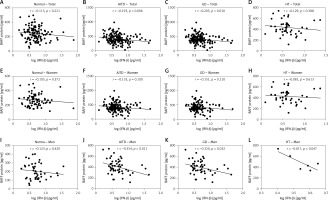
There were no significant associations of IFN-β levels with BAFF in normal subjects or all HT patients (r = –0.113, p = 0.221 and r = –0.129, p = 0.388, respectively; Figures 4 A, D). In the meantime, there were significant negative associations of IFN-β levels with the serum BAFF protein in all AITD and GD patients (r = –0.193, p = 0.006 and r = –0.203, p = 0.010, respectively; Figures 4 B, C). There were no significant correlations between serum IFN-β and BAFF protein levels among women in the control, AITD, GD, or HT groups (Figures 4 E–H). On the other hand, in men, no significant correlation was observed in the normal controls (Figure 4 I). However, there were significant negative correlations between serum IFN-β and BAFF protein levels in men in the AITD, GD, and HT groups (r = –0.354, p = 0.011, r = –0.320, p = 0.032, and r = –0.817, p = 0.047, respectively; Figures 4 J–L).
Associations of smoking with IFN-β, TSHRAb, and BAFF levels
As the percentage with a smoking habit in men was higher than that in women, we further analyzed the associations of a smoking habit with IFN-β and TSHRAb levels, and associations of a smoker’s IFN-β and serum BAFF levels in all, female, and male GD patients using Pearson’s correlations. The data showed that a smoking habit was not correlated with TSHRAb or BAFF levels in all, female, or male GD patients (Supplementary Table SV). Moreover, we further evaluated the association of IFN-β with TSHRAb and BAFF levels in non-smokers and smokers (Supplementary Table SVI). Interestingly, we found that in the smoker group, IFN-β was inversely correlated with TSHRAb and BAFF levels in all (r = –0.600, p = 0.005, and r = –0.478, p = 0.003, respectively) and male GD patients (r = –0.788, p < 0.001, and r = –0.562, p = 0.002, respectively), while no significant correlations between IFN-β and either TSHRAb or BAFF were observed in female GD patients. On the other hand, there were no significant associations of IFN-β with TSHRAb or BAFF levels in non-smokers. Finally, no significant associations of IFN-β levels with BAFF levels were detected in all, female, or male normal subjects.
Discussion
Data in this study showed that serum IFN-β levels were lower in patients with HT and AITD than those in the controls in an ethnic Chinese (i.e., Taiwanese) population. There was a gradually decreasing percentage of high IFN-β levels across the spectrum of GD without AmiA, to GD with AmiA and HT, which implies that gradually downregulated IFN-β levels could progressively promote thyroid autoimmunity until the occurrence of autoimmune thyroiditis. Despite the lack of associations of serum IFN-β levels with GD, TSHRAb levels declined with increments of serum IFN-β levels in men but not in women. Meanwhile, BAFF levels also dropped with elevated serum IFN-β levels only in men with GD, HT, and AITD. These results suggested that in men with GD, IFN-β could repress serum TSHRAb production and restrict BAFF levels after disease onset. The results provide important information on the potential role of IFN-β in the pathogenesis of AITD. Restoring the serum IFN-β level might be beneficial in preventing the development of HT. On the other hand, it might be advantageous for men with GD to replace IFN-β to suppress BAFF and TSHRAb.
Both IFN-β and IFN-α bind to the same INF receptor and exert similar antiviral functions [20]; however, different biological activities exist. For instance, IFN-β has a stronger binding affinity to the receptor, and binds the receptor for a longer time, which could induce differential antiviral and immune-related gene expression, and subsequently lead to different antiviral properties and levels of immunity [21, 22]. In addition, IFN-α is more prone to enhancing systemic inflammation and autoimmunity, while IFN-β can reduce local inflammation [23]. More importantly, IFN-β was shown to restrain antigen presentation in antigen-presenting cells (APCs), inhibit T-cell activation, alter the proinflammatory and inflammatory cytokine balance, and ultimately restore the immunosuppressive function, which could impede the occurrence of AIDs [7]. Therefore, compared to IFN-α, IFN-β is considered to exert a protective role in the occurrence of autoimmunity [24]. Thus, the occurrence of AIDs can be expected as a result of an impaired IFN-β signature. Feng et al. reported that compared to the systemic AID of SLE, type 1 IFN activity was downregulated in patients with MS, an organ-specific AID [5]. Moreover, Teige et al. observed that IFN-β-deficient mice were vulnerable to the occurrence of experimental autoimmune encephalomyelitis, and exacerbated central nervous system inflammation, which further highlighted the immunosuppressive role of IFN-β [25]. In the current study, we found reduced serum IFN-β levels in HT patients. To confirm our data, IFN-β, together with age, sex, and FH, which were shown to be significant predictors in the development of HT by a univariate analysis, were further analyzed by multivariate logistic regression. IFN-β was still retained in the model (p = 0.043, OR = 0.20, 95% CI: 0.04–0.95, data not shown) for predicting the occurrence of HT. The findings suggested that IFN-β is an independent contributor to the development of HT. In addition, IFN-β levels decreased in the progression from GD without anti-TPO, to GD with anti-TPO and HT, all of which suggests that IFN-β dysregulation could participate in the pathogenesis of AITD. However, the actual mechanism of action of IFN-β in HT remains to be determined, and further molecular-based research is required.
It is known that IFN-β can modulate B-cell function through T cell-dependent or -independent mechanisms [15, 26, 27]. In addition to cell-mediated immunity, an exaggerated humoral immune response and autoantibody formation were also observed in MS, which suggests that an impaired IFN-β signature might also be linked to pathogenic B-cell activation [28, 29]. In the present cross-sectional study, we were unable to confirm the presence of a significant association of IFN-β levels with GD. This could possibly be partially attributed to almost all GD samples in the present study being collected from patients receiving ATD, which exerts a remarkable immunoregulatory effect [30], and subsequent changes in IFN-β levels would be anticipated. Interestingly, the serum IFN-β level was negatively associated with the serum TSHRAb level at enrollment in men with GD, which still implies a possible immunomodulatory role of IFN-β in TSHRAb production after GD develops. Moreover, a correlation between serum IFN-β and TSHRAb level was observed only in men and not in women, which suggests that sex steroids are also implicated in the regulation by IFN-β of TSHRAb production. Despite the lack of direct evidence demonstrating the significant association with gender of peripheral B cell counts, several studies have shown that differential B-cell subpopulations, global gene expression of B-cells, immunoglobulin levels, the capacity for antibody production after exposure to antigens, and divergent autoimmunity exist between women and men [31–33].
BAFF is known as a crucial player in B-cell development and differentiation, and antibody formation, and recent evidence also showed that BAFF offers T cell co-stimulation and enhances interactions between APCs and T-cells [34]. As mentioned above, in addition to the cardinal mechanism of T-cell-mediated immune responses in MS, upregulation of the endogenous BAFF level and serum autoantibody production were detected in MS [35]. Elevated BAFF levels together with a diminished endogenous IFN-β signature in drug-naive MS patients suggests a possibly repressive effect of endogenous IFN-β on BAFF activity through uncertain mechanisms [5, 27]. In contrast, Krumbholz et al. reported that administration of exogenous IFN-β in cultured stromal cells and immunocytes promoted BAFF secretion [15]. At the same time, they also observed that exogenous IFN-β priming increased serum BAFF protein levels in MS patients [15]. In our previously published research, serum BAFF levels were higher in GD and HT, and associations of serum BAFF with the thyroid autoantibody were only observed in women and not in men, which indicated that sex steroids possibly modulated BAFF’s function [14]. In the present study, we observed no correlations between serum BAFF and endogenous IFN-β in healthy controls, while serum IFN-β levels were negatively correlated with BAFF levels in men with either GD or HT. Collectively, we speculated that in normal healthy subjects, IFN-β and BAFF are physiologically maintained at basal levels in a homeostatic state. After the onset of GD or HT, it appears that the dysregulated IFN-β signature fails to restrain BAFF’s function, which subsequently leads to elevated BAFF activity. Moreover, correlations between IFN-β and BAFF levels differed by gender, which also implied that testosterone and estrogen might play differential roles in IFN-β’s modulation of BAFF’s function. Evidence showed that estrogen upregulates while testosterone inhibits BAFF gene expression [36, 37]. We assumed that in women, the effect of endogenous IFN-β limiting BAFF production was weakened by estrogen, which is known to enhance BAFF’s function, and subsequently resulted in the absence of significant associations between IFN-β and BAFF levels in women. In contrast, in men, the significant association of IFN-β with TSHRAb levels might be attributed to the prominent effect of IFN-β regulating BAFF’s function. On the other hand, in addition to being a known risk factor for inducing autoimmunity, smoking was also reported to suppress BAFF and antibody formation [38–40]. Thus, one may question whether the specific associations of INF-β with TSHRAb and BAFF in male patients were due to there being more smokers among males. Interestingly, our results showed that although no significant association of a smoking habit with IFN-β, TSHRAb, or BAFF was detected in all, male, or female patients with GD, in smokers, IFN-β was inversely correlated with TSHRAb and BAFF levels in all and male patients with GD. The above observations suggest that a smoking habit might not directly influence TSHRAb or BAFF titers in GD, but might indirectly participate in IFN-β’s regulation of TSHRAB and BAFF levels. However, additional studies are required to clarify this hypothesis.
In conclusion, to the best of our knowledge, this is the first study to demonstrate downregulation of serum IFN-β protein levels with HT, which is, in essence, a cell-mediated AID. Moreover, we also found that IFN-β levels were inversely correlated with TSHRAb levels at enrollment in men with GD, and with serum BAFF levels in men with GD, HT, or AITD. However, the present study has several limitations. First, our sample size of HT patients was relatively small; an additional study with a larger sample size would make our results more convincing. Second, it was documented that a previous pregnancy could induce future maternal autoimmunity [41]. However, the pregnancy history was not recorded in the present study, and thus we could not identify the influence of a prior pregnancy on autoimmunity or IFN-β levels. Third, most blood specimens of patients with AITD were not drug naïve at enrollment (GD drug naïve, n = 9), so alterations in IFN-β activity compared to those prior to ATD or levothyroxine were anticipated. Collecting and quantifying IFN-β in the initial status prior to medications and comparing them to IFN-β in a post-treatment status in a longitudinal study would make our observations more reliable. Finally, this was an observational study, and the actual mechanisms behind these findings are unclear. Future well-designed in vivo and in vitro studies that investigate the mechanism of IFN-β in initiating or promoting AITD, and the association of IFN-β with BAFF levels, are required.