Systemic mastocytosis (SM) is a malignant hematological condition characterized by abnormal mast cell proliferation and accumulation of the extracutaneous organs, including the gastrointestinal tract, bone marrow, liver, and spleen [1, 2]. According to the World Health Organization’s (WHO) classification of hematolymphoid neoplasms, systemic mastocytosis is divided into five categories: indolent SM (ISM), SM with an associated hematological neoplasm (SM-AHN), smoldering SM (SSM), aggressive SM (ASM), and mast cell leukemia [3].
The primary diagnostic criterion for SM is the presence of a multifocal dense mast cell aggregate (> 15 cells) in the bone marrow or extracutaneous biopsies. The additional minor criteria are: 1. Expression of CD2, CD25, or CD30 by flow cytometry or immunohistochemistry; 2. KIT activating mutation(s) at codon 816 or in other critical regions of KIT; 3. > 25% of the mast being spindle-shaped or having atypical embryonic morphology, 4. Serum total tryptase > 20 ng/ml. The diagnosis of SM may be considered when the main criterion along with one minor criterion is met or three minor criteria are met [4–6].
The diarrhea-predominant subtype of IBS is characterized by frequent episodes of diarrhea preceded or accompanied by abdominal discomfort in the patient. Over 70% of patients with systemic mastocytosis report diarrhea as one of the most prevalent symptoms. Despite this, diarrhea is a common clinical symptom that can be caused by several factors that may lead to mastocytosis-related diarrhea in the future [4, 6].
This review aims to elucidate the main characteristics of GI involvement caused by systemic mastocytosis, as illustrated by a case study involving a patient initially diagnosed with refractory IBS-D.
Pathogenesis of SM
Paul Ehrlich first identified mast cells as separate inflammatory mediator cells in 1878 due to special cytoplasmic and metachromatic granules in them. A range of vasoactive biological mediators, including histamine, proteolytic enzymes including leukotriene, tryptase, prostaglandin, heparin, and tumor necrosis factor (TNF), are released when the mast cells degranulate [6].
The molecular pathophysiology of SM is not entirely understood; however, 90% of cases are linked to KIT genetic defects, most frequently the KIT D816V mutation. The stem cell factor (SCF) tyrosine kinase transmembrane receptor is known as KIT. The signal transduction pathway that causes mast cell proliferation, differentiation, maturation, survival, migration, and cytokine generation is phosphorylated by KIT upon activation. The KIT D816V point mutation causes the tyrosine kinase receptor to become constitutively activated at position 816 by changing the amino acid from aspartic acid to valine [7].
Clinical features of SM
Unlike cutaneous mastocytosis, SM predominantly affects adults, with a median age of 43. The acute and chronic discharge of mast cell mediators fundamentally causes mastocytosis clinical manifestations. Among the warning signs and symptoms are hypotensive shock and skin manifestations, including urticaria, pruritis, and pigmentosa. GI symptoms, particularly nausea, abdominal distress, and diarrhea, are frequently reported in over 80% of SM patients, regardless of the presence or absence of direct mast cell infiltration of the mucosa [6].
Numerous clinical manifestations have been linked to SM, ranging from modest constitutional symptoms to potentially fatal manifestations associated with mediator-mediated end-organ damage. A new medication or insect bite can induce anaphylaxis, a dangerous SM side effect resulting in syncope, hypotension, laryngeal edema, and sudden cardiac collapse. Similarly, there can be variations in the clinical forms, ranging from SM with mild symptoms to mast cell leukemia. Due to this heterogeneity, it may be difficult to diagnose the condition, delaying therapeutic intervention [8].
Diarrhea predominant irritable bowel syndrome (IBS-D)
IBS, a common functional gastrointestinal disorder with no known organic pathology, is characterized by persistent abdominal pain and bowel pattern disturbances. Three primary classifications for IBS have been identified based on the predominant gastrointestinal habit: mixed IBS, IBS with diarrhea, and IBS with constipation [9].
Recent research has investigated the function of mast cells in diarrhea-predominant irritable bowel syndrome (IBS), a disorder that has markedly improved in certain patients after treatment with a mast cell mediator release inhibitor (cromolyn) [10].
Involvement of clonal mast cells in the gastrointestinal tract in SM can result in significant alterations in bowel habits and IBS-D. Mast cell aggregation in the gastrointestinal mucosa, even in the absence of SM, is one of the causes of diarrhea. Prostaglandin E2 (PGE2) and histamine released by adjacent or distant mast cells increase intestinal motility and gastric hypersecretion. In addition, elevated blood levels of histamine can exacerbate GI dysfunctional symptoms such as dyspepsia, nausea, vomiting, and abdominal pain by increasing the risk of duodenal ulceration, peptic ulcer, and duodenitis. In cases of severe disease, the mucosal invasion by clonal mast cells can result in malabsorption and weight loss [11].
Case report
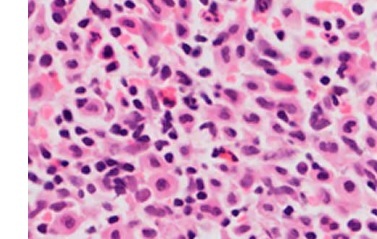
Two years ago, the patient, a 65-year-old woman, had diarrhea, dyspepsia, and abdominal discomfort. Numerous upper and lower endoscopies were performed after numerous consultations with gastroenterologists, but the results were inconclusive. Due to the inclusion of inflammatory bowel disease in the differential diagnosis, upper gastrointestinal endoscopy and colonoscopy were recommended. The results were inconsistent with endoscopic criteria for inflammatory bowel disease, celiac disease, or microscopic colitis. After being diagnosed with diarrhea-predominant irritable bowel syndrome (IBS-D), she was committed to a tertiary hospital and treated by a specialized GI team, during which a gastrointestinal biopsy was performed. Tryptase and CD117-positive mast cells with diffuse infiltration in the lamina propria were discovered during the colonoscopy (Figure 1). Due to suspected mast cell disease, the patient was referred to the hematology department for further evaluation. The bone marrow aspirate and biopsy revealed multifocal dense mast cell infiltrates positive for CD2, CD25, and tryptase by flow cytometry and immunohistochemistry (Figures 2 and 3). Notable findings included a positive c-KIT D816V mutation test and a tryptase level above 200 ng/ml. The patient was diagnosed with systemic mastocytosis and was treated appropriately.
Figure 1
A – Hematoxylin and eosin staining of the colon biopsy showing diffuse infiltration of mast cells in the lamina propria, which are positive for tryptase (B) and CD117 (C); original magnification 100×
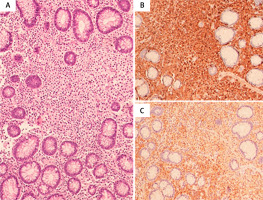
Figure 2
A – BM trephine biopsy showing hypercellularity with diffuse infiltration by spindle-shaped mast cells (H&E 40×), positive for tryptase (B). C – BM aspirate showing mast cells
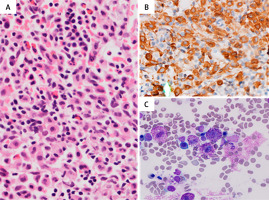
Figure 3
Flow cytometry immunophenotyping on BM aspirate showing mast cells with high side scatter and positive for CD45 (A), CD117 (B), CD25 (C), CD2 (D) and CD33 (E)
The patient commenced supportive treatment and was enrolled in a midostaurin study. She received 100 mg orally twice daily with regular monitoring for toxicity. After 3 months of medication, the patient’s symptoms significantly improved, including eliminating diarrhea and abdominal discomfort. The persistence of the underlying disease was demonstrated by detecting clonal mast cells in the gastrointestinal tract following a second biopsy. Despite this, the patient’s clinical symptoms had significantly improved.
Diagnosis
Diagnosis of SM can be challenging due to its diverse clinical features and the lack of awareness among general practitioners. While some patients with indolent SM may have a normal life expectancy, others with aggressive SM, such as mast cell leukemia, may have a survival rate of less than a year. Aggressive SM is usually associated with signs of bone marrow failure, liver dysfunction, portal hypertension, pathological fractures, and malabsorption [12].
Given the rarity of SM, its clinicopathological features can be easily overlooked. Diarrhea is one of the most common symptoms of SM, affecting more than 70% of patients. However, diarrhea is a general gastrointestinal symptom attributed to many common causes, which can delay the diagnosis of SM-related diarrhea. Additionally, SM can evade detection through standard endoscopy and colonoscopy procedures if there is no clinical suspicion. The most common endoscopic findings associated with SM are esophagitis, duodenal folds, peptic ulcers, thickened gastric walls, and dilated intestinal loops. However, the endoscopic morphology is generally nonspecific, and mast cell aggregation can be subtle and focal. Diagnosis requires an adequate biopsy and a high level of suspicion among pathologists and doctors [13].
Histology of SM in GI biopsy
Gastrointestinal manifestations of SM are increasingly recognized as GI biopsies become more common in clinical practice. However, the histological criteria for SM in GI mucosa biopsy remain controversial [14].
According to Doyle et al., the ileum is the most common site involved in SM (86%), followed by the colon (81%), the duodenum (67%), and the stomach (35%). They also suggested that intestinal biopsies could be an excellent alternative to bone marrow specimens for identifying mast cell infiltrates, defined as ≥ 15 mast cells in aggregates [15].
Mast cell infiltrates in GI are predominantly subepithelial clusters of spindle-shaped or ovoid cells with abundant eosinophilic cytoplasm involving the lamina propria. The infiltration pattern can be diffuse, multifocal, or even a single discrete collection of mast cells. In some patients, mast cell infiltrates may be surrounded by dense clusters of eosinophils, obscuring the mast cells and leading to misdiagnosis as eosinophilic colitis [16].
Immunohistochemical stains for CD117 and tryptase help highlight mast cells in both reactive and neoplastic conditions. CD117 is more sensitive, while tryptase is more specific. Mast cell atypia can be determined through CD2, CD25, and CD30 expression. Only CD25 positivity is considered the hallmark of atypical mast cells, as CD2 is less sensitive, and CD30 is expressed mainly in advanced SM or mast cell leukemia [16, 17].
According to Doyle et al., mast cells in liver biopsies from patients with SM are characteristically spindle-shaped, surrounded by fibrosis, and show both sinusoidal and portal involvement.
Molecular testing
KIT D816V is a somatic mutation that drives the development of SM. It plays a major role in SM diagnosis, therapy, and overall disease prognosis [6, 17]. KIT D816V mutation is considered a minor criterion for SM diagnosis and is found in over 90% of SM patients. However, aggressive forms of SM, such as mast cell leukemia, have a lower prevalence of KIT D816V (40–67%) and are more likely to harbor atypical mutations, such as D816H/Y or F522C [4, 18].
Multi-lineage involvement by the KIT mutation is related to disease progression, increased organ dysfunction, and high disease burden. The presence of multiple concurrent mutations, such as ASXL1, RUNX1, SRSF1, and NRAS, is linked to SM-associated myeloid neoplasms. In aggressive forms of SM, the presence of SRSF2/ASXL1/RUNX1 mutations is an independent predictor of poor survival [4].
Serum biomarkers
In recent years, several serum biomarkers for mastocytosis (SM) have been identified with diagnostic and therapeutic potential. These markers are chemicals that mast cells produce or are related to mast cell activity.
The most well-known marker is serum tryptase, a minor criterion for SM diagnosis and essential in disease monitoring. Other biomarkers include urinary histamine metabolites, IL-6, IL-31, carboxypeptidase A, the checkpoint molecule PD-1, ligand PD-L1, neuropeptides, and bone cytokines [19].
PD-1 (programmed death-1) and its ligands PD-L1 and PD-L2 are immune checkpoint molecules that suppress active immune cells to prevent autoimmunity. However, in tumors, these molecules can also assist in immune evasion, allowing cancer cells to escape attack by the immune system. Recent studies show that serum PD-L1 levels are significantly higher in SM patients than in healthy controls. Additionally, PD-L1 expression was increased on neoplastic mast cells but not on other neoplastic or reactive bone marrow cells. PD-L1 can be assessed by immunohistochemistry (IHC), flow cytometry, and serum level [20–23].
Overall, these biomarkers are still under investigation, but they have the potential to revolutionize the diagnosis, treatment, and monitoring of mastocytosis [22].
Treatment of SM
SM is a complex and challenging disease. Treatment is tailored to the individual patient’s disease characteristics and symptoms. The triggers of MC degranulation, such as insect bites, emotional stress, infections, and allergies, must be avoided [5].
Symptomatic treatment to control MC mediator-related features includes H2 blockers, antihistamines, leukotriene antagonists, cromolyn sodium, and anaphylaxis treatment. Histamine H2 receptor antagonists and proton pump inhibitors are the drugs of choice to manage GI-related symptoms. Horan et al. concluded that cromolyn sodium was an effective medication to reduce diarrhea, nausea, and abdominal pain, and hence can be considered as second line therapy [10].
Three tyrosine kinase inhibitors (TKIs), imatinib, midostaurin, and avapritinib, have been approved as cytoreductive drugs for aggressive SM treatment to limit the disease burden and progression. Recent studies have proved the efficacy of TKI as a disease-modifying therapy, achieving a deep molecular response regardless of the SM subtype, prior therapy, and mutational status. Imatinib is reserved only for atypical KIT mutations and has a limited role against KIT D816V [24–26]. Midostaurin, a multitargeted TKI, has been approved for the treatment of aggressive forms of sickle cell disease (SM), such as mast cell leukemia (MCL). Regarding diminishing serum tryptase levels and the number of mast cells in the bone marrow, it has a response rate of approximately 60%, demonstrating its efficacy against the KIT D816V mutation and FLT3. Adverse effects such as nausea, vomiting, peripheral edema, and QTc prolongation are typical [26]. Avapritinib, a potent inhibitor of platelet-derived growth factor receptor α (PDGFRA) and tyrosine kinase (TKI), was recently approved by the US Food and Drug Administration for the treatment of advanced SM. DeAngelo et al. found that avapritinib had an overall response rate of 75% and a complete remission rate of 36% in patients with advanced SM. Due to the 13% of patients who encountered cerebral hemorrhage, its use in thrombocytopenia patients is limited. The most common side effects of avapritinib include periorbital edema, cognitive changes, thrombocytopenia, and anemia [27]. Cladribine was commonly used before TKI inhibitors and still has an added advantage against SM-associated neoplasm and debulking of the disease prior to allogenic stem transplantation [24].
Conclusions
Systemic mastocytosis must be an important clinical consideration in patients with unexplained gastrointestinal symptoms, especially when associated with refractory features or elevated serum tryptase. Early diagnosis can be achieved through a high clinical index of suspicion among general practitioners, gastroenterologists, and pathologists.