High mortality from gastric cancer (GC) is a serious challenge to global health, especially in East Asia and Eastern Europe, where GC remains one of the leading causes of cancer-related deaths [1]. Early detection of GC tends to improve treatment efficiency and survival rates. Currently available early detection methods mainly involve endoscopic biopsy, which is considered the gold standard of diagnosis. Other than these, noninvasive techniques include a serum pepsinogen test and a Helicobacter pylori (Hp) test as a screening technique for the high-risk population. Recently, ways have critically emphasised some molecular markers based on methylated DNA with specific microRNAs presented in blood and gastric juice, which are expected to give better rates of early detection [2]. Given their detectability in gastric mucosal carcinogenesis, methylated SEPT9 and ring finger protein 180 (RNF180) are acquiring prominence in the field of GC early diagnosis. The SEPT9 gene is already known to deal with the cytoskeletal tissue, and its methylation was considered to have a bright future in the blood-based detection methodology that shall not be invasive for the screening of GC [3]. Also, the RNF180 gene, encoding an E3 ubiquitin-protein ligase, also shows methylation changes in gastric tissue detection in tissue samples and blood [4]. The detection of combining these 2 biomarkers is thought to increase the accuracy of diagnosis, providing options for the early detection and management of GC [5]. Therefore, we seek to determine their effects in diagnosing GC patients in a Chinese population and compare diagnostic performance across all gastric diseases and cancer stages.
This was a retrospective study. The study involved the retrieval of stored blood samples and medical documentation from individuals who had undergone endoscopic biopsy for early GC screening between January 2021 and December 2023. High-risk subjects were defined as aged ≥ 40 years, resident in regions where the incidence of stomach cancer is high. Those with Hp and those who had a previous history of pre-cancerous gastric conditions like atrophic chronic gastritis, gastric ulcer, gastric polyp, or pernicious anaemia were included. This study followed the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guideline.
The clinical data obtained encompassed demographic details such as age and gender, as well as comprehensive clinical characteristics including the type and location of gastric lesions, Lauren classification, TNM staging, and lymph node metastasis status. Additionally, we quantified key tumour markers using the Roche E-70 analyser. To differentiate between benign and malignant gastric conditions, specific cut-off values were established: ≥ 5 ng/ml for CEA, ≥ 37 U/ml for CA19-9, and ≥ 6.9 U/ml for CA72-4.
Because this SEPT9/ RNF180 methylation detection process uses a fixed approach, processes were conducted under fixed circumstances to control and maintain the validity and reliability of the results. In line with this, 10 ml blood samples were drawn up in K2EDTA anticoagulant tubes from a BD Vacutainer® to avoid clotting and centrifuged for 12 min under 1350 ±150 rpm so that the plasma could separate. If not to be immediately processed, the plasma was stored between 2°C and 8°C. In detail, plasma was centrifuged for the second time under the same conditions, 3.5 ml aliquots were placed in labelled conical tubes, and then they were stored at –25 to –15°C for DNA preservation for a period of 2 weeks.
DNA methylation was assessed using a two-step process employing the PCR fluorescence probe technique. Initially, free DNA was isolated from plasma and treated with bisulfite to convert unmethylated cytosines to uracil sulfonate, leaving methylated cytosines unaltered. Subsequently, triplex PCR amplification was conducted, in which specific PCR blockers and fluorescent probes identified and amplified methylated sequences of the RNF180 and SEPT9 genes. Each sample, along with positive and negative controls, was processed in parallel to ensure consistency.
These statistical analyses were performed with Python 3.12. The receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were calculated to assess the performance of the different biomarkers as a diagnostic test. Estimation of confidence intervals for sensitivity, specificity, positive predictive value, and negative predictive value was done via the efficient score method as provided in statsmodels Wilson score interval.
In this study, 8 hospitals in Yunnan Province were selected. The blood samples of 201 patients who underwent endoscopic biopsy for early GC screening were retrospectively collected from these hospitals from January 2021 and December 2023: 78 with GC (GC+) and 123 without GC (GC–). The GC+ group had a mean age of 60.2 years (SD ± 5.3), whereas the GC– group had a mean age of 57.6 years (SD ± 5.9). The baseline characteristics are summarised in Table I.
Table I
General characteristics of study population
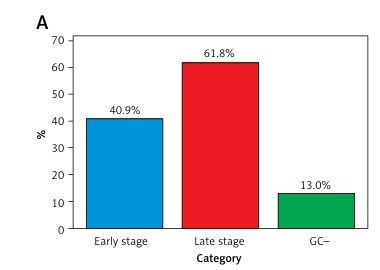
Figure 1 A revealed that methylated SEPT9 and RNF180 had varying positive detection rates across cancer stages, with 43.2% in early stages and 58.8% in late stages. In non-cancerous cases, these markers demonstrated a positive detection rate of 13.0%. Figures 1 B and 1 C present ROC curves quantifying the opportunity of SEPT9/ RNF180 detection by different GC groups and markers. Figure 1 D displays the ROC curves for detection using different combinations of biomarkers. Various characteristics of GC were assessed for its favourable detection rates, presenting a significant difference by the type and location of GC (Figure 1 E). The ROC curve categorised by age and gender highlights differences in diagnostic performance (Figure 1 F).
Figure 1
Diagnostic value of methylated SEPT9 and RNF180 for gastric cancer. A – Positive detection rates of methylated SEPT9 and RNF180 in participants at different stages. B – ROC curves for SEPT9/RNF180 detection. C – ROC curves for various gastric cancer markers. D – ROC curve comparison of combined biomarkers. E – Positive detection rates for gastric cancer characteristics. F – ROC curves by age and gender
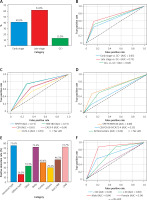
The findings from this study underscore the significant potential of methylated SEPT9 and RNF180 as biomarkers for the detection of gastric cancer. The study demonstrates that both biomarkers exhibit high specificity and improved detection rates in later stages of the disease, with the positive detection rate escalating from 43.2% in early stages to 58.8% in advanced stages of gastric cancer. The detection results of different biomarker combinations revealed that the combination of SPET9 and RNF180 achieved the highest diagnostic accuracy. Notably, the intestinal type of gastric cancer demonstrated the highest detection rate at 75.6%, indicating that these biomarkers are particularly effective in identifying more aggressive forms of the disease. Furthermore, the study highlights a clear distinction in diagnostic performance based on patient demographics, with males and older individuals showing significantly higher detection accuracies, which could inform tailored screening approaches in clinical settings.
Utilising peripheral blood for cell-free DNA (cfDNA) methylation testing has emerged as a pivotal non-invasive diagnostic method for early cancer detection. Studies have shown that methylation variations in colorectal cancer tissues may indicate the potential effectiveness of SEPT9 methylation in peripheral blood for early CRC screening [6]. This came about with the ‘Shield’ early screening tool that applies changes in cfDNA genomic variations and DNA methylation status and fragmentation patterns for colorectal cancer. The recently completed ECLIPSE study reported sensitivity and specificity of diagnosing CRC at 83.1% and 89.6%, respectively [7].
Further studies have revealed that high RNF180 and SEPT9 methylation in the plasma samples of patients with gastric cancer were sound and reasonable biomarkers for that neoplasm [8]. RNF180 is a tumour suppressor gene that suppresses gastric cancer cells’ growth, proliferation, and migration. Methylation in its promoter region, however, is positively associated with cancer cell growth, differentiation, and metastasis. RNF180 is one of the hypermethylated genes detected in the plasma of people with gastric cancer. Similarly, the SEPT9 gene, another tumour suppressor, regulates apoptosis and cell division. The sensitivity and specificity of SEPT9 methylation for diagnosing gastric cancer were 47.7% and 92.3%, respectively. However, for complex gastric lesions, these findings fell short of the required diagnostic accuracy. Combining methylated SEPT9 with protein markers could significantly improve diagnostic sensitivity [9].
Our results agree with the fact that DNA methylation markers, being ideal gastric adenocarcinoma biomarkers, are more sensitive than traditional protein tumour markers such as CEA, CA199, and CA125 for gastric cancer detection [10]. Notably, studies have demonstrated that DNA methylated biomarkers such as SEPT9 and RNF180 are particularly effective in distinguishing between malignant and non-malignant gastric tissues with high specificity. This supports our findings, which indicate specificity rates exceeding 85% for both biomarkers [11]. In addition, the differential performance of each biomarker across various cancer stages and types in our study concurs with Xu et al., who found enhanced detection in more advanced stages of gastric cancer [12]. Our work is new to the literature as we quantitatively compared the different detection capabilities according to patients’ demographics, showing significant variations likely to make a big difference in screening strategy.
Nevertheless, several limitations deserve consideration. The small sample size, drawn from only 8 hospitals in a single geographic region (Yunnan, Southwest China) limits the generalisability of the findings to broader populations. In contrast, blood-based biomarkers, which are less invasive to patients compared to tissue biopsies, may carry far less assessment in the whole heterogeneity of gastric cancer compared to tissue research, which may affect the sensitivity and specificity in different subtypes and at various stages of the tumour. Furthermore, it failed at this stage to engage in a longitudinal follow-up of this biomarker appraising its prognostic value.
In conclusion, we found that methylated SEPT9 and RNF180 are effective biomarkers for the detection of GC, especially in advanced stages and specific GC types. Their incorporation into clinical practice has the potential to improve early detection and patient prognosis in high-risk populations. However, this conclusion requires further confirmation from clinical studies with larger samples.