The most common disease among elderly patients is osteoporosis (OP), a bone disease caused by reduced bone strength and increased risk of fractures [1]. Postmenopausal osteoporosis (PMOP) is prevalent in women and is characterized by the systemic impairment of bone mass and microarchitecture. The cause of OP has not yet been fully elucidated, and many risk factors are associated with the disease, including reduced bone mass, hormonal imbalance, use of certain drugs (such as glucocorticoids), smoking, low physical activity, and insufficient intake of calcium and vitamin D [2]. Radiologic laboratory assessments (dual-energy X-ray absorptiometry (DEXA)) of bone mineral density (BMD) can accurately diagnose OP before a fracture occurs. However, DEXA scanning is costly and requires specialized equipment and trained staff [3]. Studies have shown that the change in levels of bone turnover markers (BTM) often precedes BMD and may be detected a few months after anti-OP treatment, providing valuable information regarding the diagnosis of OP and efficacy of treatment [4]. Investigations using osteoporotic serum have suggested several biomarkers for bone resorption and formation that are related to BMD. The estrogen receptor (ER) gene, vitamin D receptor VDR gene, interleukin-6 (IL-6), bone sialoprotein, procollagen type I N propeptide (PINP), and microRNAs have been investigated for their impact on BMD and their use in the diagnosis of OP [5–7]. Although BTMs, osteocalcin (OCN), cathepsin K (Cat K), and osteoprotegerin (OPG), have been shown to play important roles in bone metabolism [8–10], it is not clear whether these markers can be used to accurately predict primary PMOP in middle-aged and elderly postmenopausal women.
In this study, we measured serum levels of OCN, Cat K, and OPG in middle-aged and elderly postmenopausal women and assessed the diagnostic value of these markers for PMOP to develop alternative tools for the prevention and treatment of OP-related fragility fractures.
Methods
Women who participated in a regular OP screening program at our hospital between February 2020 and March 2022 were selected for this study. The participants were grouped into control (non-OP), osteopenia (BMD T-scores –2.5 to –1.0), and OP (T-scores (< –2.5) groups [11]. The participants were included if they had menopause for at least 1 year and were aged between 50 and 75 years. Participants were excluded if they had fractures, malignant tumors, dysfunction of important organs such as the heart, liver, and kidney, or diseases such as hepatitis and uremia. Participants were also excluded if they took medications affecting bone metabolism and hormone balance. Participants who had received bone disease-related treatments were also excluded.
All participants were subjected to DEXA scanning, and BMD was measured using a Discovery Wi DEXA Machine (Hologic, USA) according to the manufacturer’s protocol. Fasting venous blood was drawn from all participants into 5-ml vacuum containers with added potassium-EDTA as an anticoagulant within two days of DEXA scanning. An Agilent 2100 Bioanalyzer was used to analyze the samples based on luminescent immunoassays using the double-antibody sandwich technique. Enzyme-linked immunosorbent assay (ELISA) kits for human OCN (Cat. no. BMS2020INST), and Cat K (cat. no. EEL033) and OPG (cat. no. BMS2021INST) were purchased from Thermo Fisher Scientific (Waltham, IL, USA).
Statistical analysis
Statistical analyses were performed using SPSS version 18 software package for Windows (SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov analysis was performed to evaluate whether the distribution of values was normal. The measurement data are expressed as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used for intergroup comparisons. Pearson’s correlation analysis was performed to assess the relationships between the parameters. Multiple logistic regression analyses were performed to determine risk factors for PMOP. The diagnostic accuracy of the PMOP markers was assessed by calculating the areas under the receiver operating characteristic (ROC) curves. All of the reported p-values were based on 2-sided tests, and those less than 0.05 were regarded as statistically significant.
Results
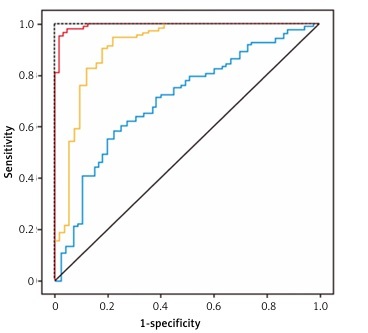
A total of 252 participants were included in this study and grouped into control (77), osteopenia (80), and OP (95) groups based on their BMD values. The participants’ characteristics are listed in Table I. The analysis showed that there were significant differences in age, BMD, serum OCN, Cat K, and OPG levels among the groups (p < 0.01). Compared with the control group, participants in the osteopenia and OP groups were significantly older, with a longer time since menopause, higher Cat K levels, and lower OCN and OPG levels (Table I). Pearson’s correlation analysis showed that participants’ BMD was negatively correlated with Cat K levels and positively correlated with OCN and OPG levels (p < 0.01). Multiple logistic regression analysis revealed that OPG was the biggest risk factor, with an odds ratio (OR) of 3.765 for PMOP among the three assessed markers when age, BMI, and diseases were included in the calculation (Table II). The ORs for OCN and Cat K were relatively low (between 1.214 and 1.420) compared with OPG. To determine whether these markers could be used to predict PMOP, ROC curves were plotted to determine the discriminative power of OCN, Cat K, and OPG for PMOP (Figure 1). The results showed that the areas under the ROC curves (AUC) for PMOP were 0.922 for OPG, 0.839 for OCN, and 0.671 for Cat K. The sensitivity, specificity, and cut-off values of these markers for predicting PMOP are listed in Table III. These results showed that among the three parameters, OPG had the highest discriminative power for OP, with high sensitivity and specificity, whereas Cat K was the least accurate marker with low sensitivity and specificity.
Table I
Comparison of parameters according to osteoporosis status
Table II
The multivariate logistic regression analysis for postmenopausal osteoporosis
| Variables | β | SE | Wald value | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| OCN | 0.388 | 1.437 | 0.114 | 1.214 (0.665–1.672) | < 0.05 |
| Cat K | 0.434 | 0.838 | 0.370 | 1.420 (0.864–1.722) | < 0.05 |
| OPG | 1.729 | 1.607 | 2.954 | 3.765 (1.965–4.612) | < 0.01 |
Table III
Diagnostic performance of serum bone markers for postmenopausal osteoporosis
| Variables | AUC | 95% CI | Sensitivity (%) | Specificity (%) | Cutoff value |
|---|---|---|---|---|---|
| OCN | 0.839 | 0.539–0.989 | 89.15 | 79.35 | 22.2 [μg/l] |
| Cat K | 0.671 | 0.519–0.755 | 63.44 | 81.41 | 7.1 [pmol/l] |
| OPG | 0.922 | 0.769–0.955 | 93.75 | 91.25 | 1.7 [μg/l] |
Figure 1
Receiver operating characteristic (ROC) curves of serum osteocalcin, cathepsin K and osteoprotegerin for prediction of postmenopausal osteoporosis in middle-aged and elderly postmenopausal women
Comprehensive screening of PMOP could serve as an important preventive strategy to reduce the risk of fragility fractures. For this purpose, we determined the levels of several bone markers and analyzed their relationship with BMD and PMOP in middle-aged and elderly postmenopausal women. Our results showed that OCN and OPG were lower, and Cat K was higher in women with osteopenia and OP. The three markers, particularly OPG, had significant predictive values for PMOP and could be used to detect and monitor the occurrence and development of PMOP. OCN is a non-collagen protein secreted by osteoblasts and is a phenotypic marker for osteoblasts. It plays an important role in bone formation and turnover processes [12]. In the present study, OCN levels were reduced in participants with osteopenia and OP and were positively correlated with BMD. Logistic analysis showed that it had an impact on PMOP, with a relatively low OR. Recent studies also showed that long non-coding RNA and microRNA may also be involved in the pathogenesis of OP [13, 14] and may therefore be explored as biomarkers for OP or therapeutic targets.
Cat K is a lysosomal cysteine protease that binds to triple-helix type I collagen, resulting in the degradation of the amino- and carboxyl-terminal cross-linked type I collagen [15]. Increased Cat K levels have been observed in patients with OP, and treatment of OP with anti-OP drugs such as alendronate significantly reduces Cat K levels [16], suggesting that Cat K is a useful indicator for monitoring the therapeutic response to anti-OP drugs. In the present study, Cat K was higher in participants with osteopenia and OP and was negatively correlated with BMD. Logistic analysis showed that it was a risk factor for PMOP with a relatively low OR.
OPG is a decoy receptor for receptor activator of nuclear factor-kappa B ligand (RANKL), which is primarily synthesized and secreted by osteoblast-lineage cells. Several studies have shown that OPG is reduced in patients with OP compared with those without no osteoporosis [17]. Our study showed that OPG was lower in participants with osteopenia and OP, and its level was positively correlated with BMD. Logistic analysis showed that it had the highest OR for PMOP among the three markers, indicating that it was a strong risk factor for PMOP.
To reduce potential bias, participants were grouped according to their T-score of BMD, obtained in the Radiology Department. Biochemistry assays were performed in hospital laboratory. The staff who made these assessments were blinded to patient information.
To determine the predictive values of these markers for PMOP, ROC curves were plotted, and analysis showed that the AUC of OCN, Cat K, and OPG to predict PMOP were 0.839, 0.671, and 0.922, respectively. Although all three markers showed a significant discrimination power for PMOP, OPG had a higher AUC, sensitivity, and specificity than OCN and Cat K, suggesting that it is highly reliable for predicting PMOP. The AUC of OPG is higher than the 0.9 threshold as a diagnostic marker with very good accuracy [18]. Therefore, OPG may be used in single-parameter tests to pre-screen women to identify possible PMOP for confirmation with DEXA scanning or anti-OP treatment.
The present study had some limitations. First, this was a retrospective study and the number of participants was low, and all participants were recruited from among visitors to our hospital and thus were not fully representative of the general population. Further studies are required to expand the sample size and include participants with diversified ethnicities. Second, only three BTMs were assessed, and it remains unclear whether there are more relevant and robust serum markers of PMOP.
In conclusion, our study demonstrated that BMD is closely related to OCN, Cat K, and OPG levels. OPG has high accuracy in predicting PMOP in middle-aged and elderly postmenopausal women and could be used as a screening tool to identify women at high risk for the diagnosis and treatment of PMOP.