β-thalassemia is a common hereditary disorder, especially in the Middle East [1], and is the most common hemoglobinopathy in Jordan with a carrier rate of approximately 2–4% [2]. Clinical classifications often focus on disease severity, and cases fall into one of three broad categories: β-thalassemia minor, β-thalassemia intermedia, and β-thalassemia major [3].
More than 300 different pathogenic variants affecting diverse levels of β-globin gene expression and β-thalassemia have been discovered [4]. However, these variants are not spread uniformly; they have geographical and racial origins, as each is distinguished by a few common variants and a varying number of unusual variants [5].
The carrier prevalence rate of thalassemia in Jordan is currently reported as around 2–4%. The number of registered cases treated by the Ministry of Health (MOH) is 656 patients (550 with β-thalassemia major and 106 with β-thalassemia intermedia). The number of patients in the four thalassemia treatment centers overseen by the MOH was 1228 according to the national registry in 2017, representing 86% of the total of 1450 thalassemia patients in the Hashemite Kingdom of Jordan [2].
Therefore, we aimed to investigate and update the prevalence of β-thalassemia variants in Jordan and compare it with other studies in Jordan and neighboring countries.
Methods
Patient cohort
The study population consisted of 158 individuals, 82 females aged 1 to 74 years with mean age of 22.39 ±16.46 and 76 males aged 1 to 80 years with mean age of 20.55 ±18.27. Amman had the highest share of β-thalassemia patients with 65.8%, followed by Irbid at 14.5%, Zarqa at 8.9%, and Ma’an at 2.5%, while Mafraq and Tafilah had 1.9% each; Aqaba, Jarash, and Madaba had 1.3% each; and Balqa had 0.6%.
The current study received ethical approval from the ethical committee and institutional review board (IRB) of Al-Balqa Applied University in Amman, Jordan (IRB number: 26/3/2/274). Informed consent was obtained in all cases.
Methods
Patients underwent hematological testing, including CBC, Hb electrophoresis, and genetic testing, to screen for β-thalassemia. Vacutainer blood samples (4 ml) containing ethylenediaminetetraacetic acid K3 (EDTA) anticoagulant (Becton Dickinson, Franklin Lakes, NJ, USA) were drawn from the patients for routine hematological investigations, including complete blood count (CBC) using Sysmex XN-1000 (Sysmex Corporation, Kobe, Japan) and Hb electrophoresis using MINICAP (Sebia, Lisses, France). DNA was subsequently extracted from the blood samples using an automated nucleic acid extraction instrument, EZ1 (Qiagen, Hilden, Germany). A dsDNA high-sensitivity assay kit was used to measure the DNA concentration in ng/μl using the QuBit 4 fluorometer, following the manufacturer’s instructions (Thermo Fisher Scientific, USA).
The isolated DNA was amplified by multiplex PCR (Veriti Thermal 96-Well Thermal Cycler Applied Biosystems, USA) using commercial β-globin strip assay, allowing the multiplication of 22 targets of β-globin and detection using reverse hybridization (Vienna Lab Labor diagnostika GmbH, Austria). The extrapolation of the results from the membrane strip was performed using StripAssay Online Calculator v2.17. Samples were processed at the hematology and genetics departments of Biolab Diagnostic Laboratories, Amman, Jordan.
Results
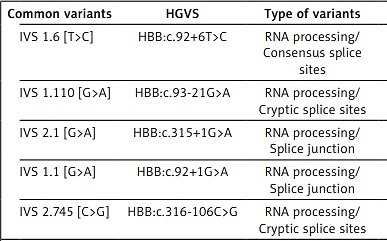
The study population was screened for 16 β-thalassemia variants, all of which were found to be β-thalassemia carriers (heterozygous mutants). No homozygous or compound heterozygous variants were identified in this study. The distribution of variants is classified by common and rare variants [6]. Sixteen different β-thalassemia variants have been reported. Five of them were represented in about 65.82% of the total Jordanian thalassemic variants and were classified as common variants: IVS 1.6 [T>C] (17.72%), IVS 1.110 [G>A] (13.29%), IVS 2.1 [G>A] (13.29%), IVS 1.1 [G>A] (11.39%), and IVS 2.745 [C>G] (10.13%). Another eleven variants were classified as rare variants and represented about 25.93% of the total Jordanian thalassemic variants and include codon 8 [-AA] (5.06%), codon 5 [-CT] (4.43%), codon 39 [C>T] (4.43%), IVS 1.5 [G>C] (3.16%), -30 [T>A] (2.53%), -87 [C>G] (1.9%) and equal frequencies for these variants: codon 6 [-A], IVS 1.130 [G>C], codon 44 [-C], IVS 2.848 [C>A] with 0.63%, -101 [C>T] with -87 [C>G] 1.9% for both. In addition, these three variants have not been previously reported in Jordan: -101 [C>T], IVS 1.130 [G>C], and codon 44 [-C]. The different thalassemia mutations observed in Jordan are listed in Table I.
Table I
Distribution of β-thalassemia variants (common and rare)
| Common variants | HGVS | Type of variants | Phenotype | Distribution | N | % |
|---|---|---|---|---|---|---|
| IVS 1.6 [T>C] | HBB:c.92+6T>C | RNA processing/Consensus splice sites | β++ | Mediterranean | 28 | 17.72 |
| IVS 1.110 [G>A] | HBB:c.93-21G>A | RNA processing/Cryptic splice sites | β+ | Mediterranean | 21 | 13.29 |
| IVS 2.1 [G>A] | HBB:c.315+1G>A | RNA processing/Splice junction | βθ | Mediterranean, U.S. Blacks | 21 | 13.29 |
| IVS 1.1 [G>A] | HBB:c.92+1G>A | RNA processing/Splice junction | βθ | Mediterranean | 18 | 11.39 |
| IVS 2.745 [C>G] | HBB:c.316-106C>G | RNA processing/Cryptic splice sites | β+ | Mediterranean | 16 | 10.13 |
| Rare variants | ||||||
| Codon 8 [-AA] | HBB:c.25_26delAA | RNA translation/Frameshift | βθ | Mediterranean | 8 | 5.06 |
| Codon 5 [-CT] | HBB:c.17_18delCT | RNA translation/Frameshift | βθ | Mediterranean | 7 | 4.43 |
| Codon 39 [C>T] | HBB:c.118C>T | RNA translation/Nonsense codons | βθ | Mediterranean | 7 | 4.43 |
| IVS 1.5 [G>C] | HBB:c.92+5G>C | RNA processing/Consensus splice sites | β+ | Asian Indian, SE Asian | 5 | 3.16 |
| -30 [T>A] | HBB:c.-80T>A | Transcriptional variants/Promoter regulatory elements | β+ | Mediterranean, Bulgarian | 4 | 2.53 |
| -87 [C>G] | HBB:c.-137C>G | Transcriptional variants/Promoter regulatory elements | β++ | Mediterranean | 3 | 1.90 |
| -101 [C>T]*ª | HBB:c.-151C>T | Transcriptional variants/Promoter regulatory elements | β++ (Silent) | Mediterranean | 3 | 1.90 |
| Codon 6 [-A] | HBB:c.20delA | RNA translation/Frameshift | βθ | Mediterranean, U.S. Blacks | 1 | 0.63 |
| IVS 1.130 [G>C]*ª | HBB:c.93-1G>C | RNA processing/Splice junction | βθ | Italian, Japanese, UAE | 1 | 0.63 |
| Codon 44 [-C]*ª | HBB:c.135delC | RNA translation/Frameshift | βθ | Kurdish | 1 | 0.63 |
| IVS 2.848 [C>A] | HBB:c.316-3C>A | RNA processing/Consensus splice sites | β+ | UB Blacks, Egyptian, Iranian | 1 | 0.63 |
HGVS – Human Genome Variation Society [1, 26]. Available at HbVar database: http://globin.bx.psu.edu/hbvar.
The percentage of β-thalassemia variants in our study differed from those provided in previously published studies in the Jordanian population and neighboring countries. Table II shows the distribution of the variants in previous studies compared with that in the current study and indicates that the common and rare variants are still in the same category but at different percentages, which means that the prevalence of β-thalassemia variants should be updated regularly to reflect the dominant variant in the current population in order to understand the community distribution for these variants. In addition, it shows the comparison of this study with other studies conducted in neighboring countries and indicates that these common variants are rare in other countries and vice versa, which means that every population has a unique distribution of variants depending on their proximity or distance from each other and the social relations between them.
Table II
Frequency of β-thalassemia alleles in the previous studies in Jordan and 12 Arab populations with Turkey compared with the current study. Saudi Arabia (SA), Turkey (TU), Lebanon (L), United Arab Emirates (UAE), Syria (S), Egypt (E), Palestine (P), Kuwait (K), Bahrein (B), Oman (O), Tunisia (T), Algeria (A), Iraq (I)
| Variant | Phenotype | This study | Study 1 [12] | Study 2 [13] | Study 3 [14] | Study 4 [15] | S [12] | I [24] | S. A [22] | P [20] | L [25] | E [21] | K [18] | UAE [19] | B [18] | O [18] | T [23] | A [23] | TU [26] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVS 1.6 [T>C] | β+ | 17.72 | 6.6 | 8.3 | 9.9 | NP** | 5 | 3.90 | 1 | 28.7 | 15.0 | 40 | 7.3 | 1.5 | – | – | 6 | 3.3 | 6.6 |
| IVS 1.110 [G>A] | β+ | 13.29 | 22 | 25 | 23 | 22 | 21 | 30.10 | 13.5 | 17.6 | 33.0 | 48 | – | 1.0 | 1.5 | 0.5 | 12.3 | 24.7 | 41.7 |
| IVS 2.1 [G>A] | βθ | 13.29 | 19.8 | 15 | 6.1 | 20 | 9 | 18.40 | 17.5 | 2.9 | 10.0 | 7 | 29 | 2.8 | 6.1 | 3.1 | – | – | 7.2 |
| IVS 1.1 [G>A] | βθ | 11.39 | 6.6 | 10 | 16.9 | 12 | 11 | 7.80 | 4 | 9.0 | 16.0 | 24 | 7.3 | – | 3 | 1 | 0.9 | 11.7 | 8.9 |
| IVS 2.745 [C>G] | β+ | 10.13 | 12 | 14.2 | 7 | NP** | 3 | – | – | 0.3 | 1.0 | 8 | – | – | – | – | – | 0.9 | 8.6 |
| Codon 8 [-AA] | βθ | 5.06 | NP** | 0.8 | 2.6 | NP** | 8 | 0.97 | – | – | 2.5 | 1 | 3 | 2.2 | – | – | – | – | 7.7 |
| Codon 5 [-CT] | βθ | 4.43 | 3.3 | 3.8 | 5.5 | NP** | 4 | 3.90 | 1 | 2.5 | 4.0 | 1 | – | 2.1 | – | 0.5 | 1.8 | – | 1.9 |
| Codon 39 [C>T] | βθ | 4.43 | 2 | 4.6 | 6.7 | NP** | 17 | 1.90 | 7.7 | 4.6 | 0.5 | 4 | 7.3 | 2.2 | 24.2 | 1 | 27 | 27.6 | 4.6 |
| IVS 1.5 [G>C] | β+ | 3.16 | 5.5 | 1.3 | 2.6 | NP** | 4 | 9.70 | 19.2 | 1.1 | 0.5 | 10 | 18.8 | 44.5 | – | 62.4 | – | 0.4 | 2.2 |
| -30 [T>A] | β+ | 2.53 | NP** | NP** | 2.3 | NP** | 2 | – | – | 2.1 | 0.5 | – | – | – | – | – | 2.6 | 0.4 | 1.0 |
| -87 [C>G] | β+ | 1.90 | 2 | 1.3 | NP** | NP** | 1 | – | 0.34 | – | 1.5 | 3 | – | – | – | – | – | – | 1.0 |
| -101 [C>T] *ª | β++ (Silent) | 1.90 | NP** | NP** | NP** | NP** | 2 | – | – | – | – | – | – | 0.2 | – | – | – | – | 1.1 |
| Codon 6 [-A] | βθ | 0.63 | 1 | 1.3 | 1.7 | NP** | – | – | – | 0.3 | – | 1 | – | – | – | – | 10.5 | 17 | – |
| IVS 1.130 [G>C] *ª | βθ | 0.63 | NP** | NP** | NP** | NP** | 1 | 0.97 | 0.67 | – | – | – | – | – | – | – | – | – | – |
| Codon 44 [-C] *ª | βθ | 0.63 | NP** | NP** | NP** | NP** | 2 | 4.90 | 2 | – | 1.0 | – | 1 | 0.4 | 4.5 | 11.1 | 4.4 | – | 1.3 |
| IVS 2.848 [C>A] | β+ | 0.63 | 2 | 1.3 | 0.6 | NP** | 1 | 2.90 | – | 2.5 | – | 9 | – | 0.4 | – | – | 0.9 | 0.4 | – |
Discussion
Few studies have been conducted on the prevalence and distribution of β-thalassemia in Jordan. Three common variants were observed during the molecular characterization of β-thalassemia in northern Jordan: IVS 1.110 [G>A], IVS 2.1 [G>A], and IVS 2.745 [C>G], in 54% of total samples. This study demonstrated the diversity of β-thalassemia alleles in North Jordan and is in line with the outcomes of the current study, in which three variants were classified as common at different percentages [7]. The common frequent alleles in Jordan are generally similar to those found in other studies in Jordan [8, 9]. If supported by future research, these findings can provide updated data on the spectrum of β-thalassemia variants in Jordan, making the study valuable and reference-worthy [10].
The types and frequencies of the common β-thalassemia variants in Jordan are generally similar to those reported in surrounding countries and have more differences from those reported in the Arabian Peninsula. Additionally, there is a difference between variants’ distribution; the IVS 1.5 [G>C] mutation, which is rare in Jordan, Syria, Lebanon, Palestine, Tunisia, Algeria, and Turkey [11], has a much higher frequency in Oman, the United Arab Emirates, Kuwait, and Saudi Arabia and constitutes the most common mutation in Oman as well as in UAE [12]. This difference could be the result of continuous links between the Jordanian population and northeastern Mediterranean countries, as compared to the geographic isolation between the Jordanian population and other Arab countries.
The IVS 1.6 [T>C] variant was found to be the most recurrent in Palestine, accounting for 28.7%, and 40% in Egypt; IVS 1.1 [G>A] ranked third among the most recurrent β-thalassemia variants in Palestine and Egypt, with 9% and 24%, respectively, which is most likely due to the close relationship between these populations in Jordan [13]. These variants are a rarity in Saudi Arabia, Bahrain, Oman, and Tunisia [12]. In the present study, the most recurrent variant was IVS 1.6 [T>C], and IVS 1.1 [G>A] ranked third among the most recurrent variants of β-thalassemia, consistently with previous findings.
IVS 1.110 [G>A] (13.29%) is ranked as the second most common variant in Jordan, but is the variant most distributed in the countries surrounding Jordan including Syria, Iraq, Lebanon, Egypt, and Turkey [8, 14]. Its neighboring countries are in agreement with the possible ancient Greek origin of this mutation [11]. Furthermore, the frequency distribution of the IVS 1.110 [G>A] variant is less than 2% in UAE, Bahrain, and Oman [12, 15].
The IVS 2.1 [G>A] variant, which is co-ranked with the IVS 1.110 [G>A] variant (13.29%) as the second most common variant in Jordan, is also the second ranked variant in Iraq, Saudi Arabia, and Bahrain. It is the most common variant in Kuwait with 29% [12]. The lowest frequency was reported in Palestine, the UAE, and Oman [12, 13, 15].
The current study reported three variants that had not been previously reported in Jordan. The first one is -101 [C>T]; this variant was reported in Syria, UAE, and Turkey. IVS 1.130 [G>C] is the second variant and has been reported in Syria, Iraq, and Saudi Arabia. The third variant is codon 44 [-C]; this variant has been reported in Syria, Iraq, Saudi Arabia, Lebanon, Kuwait, UAE, Bahrain, Oman, Tunisia, and Turkey [8, 12, 15].
In conclusion, this study compared the spectrum and distribution of various variants with other studies to determine the current prevalence of β-thalassemia variants in Jordan. The β-thalassemia variants -101 [C>T], IVS 1.130 [G>C], and codon 44 [-C] have never been reported in a Jordanian population.