Introduction
Acute appendicitis (AA) is the most common and misdiagnosed surgical cause of acute abdomen in children. Early diagnosis of AA is obligatory to avoid complications [1]. A delay in diagnosis of AA is associated with increased risk of perforation and further complications. According to some authors, AA is divided into non-complicated (phlegmonous) and complicated (gangrenous and perforated) AA. Carr [2] described phlegmonous appendicitis as neutrophilic infiltrate involving the muscularis propria, generally circumferential. Changes in mucosa include acute inflammation and often ulcerations. Gangrenous or necrotising acute appendicitis is characterised by the necrosis of the appendix wall, and patients with this complication of AA often suffer from appendix perforation if left untreated.
Appendectomy is the therapy of choice in AA treatment [3]. However, in young children the negative appendectomy rate may be as high as 50% [4]. Thus, many aspects of AA treatment remain controversial [5].
Many attempts have been made to determine ways of decreasing the negative laparotomy rate after a clinical suspicion of AA. For this reason, it would be very important to differentiate mild early appendicitis from nonspecific abdominal pain. However, despite complete clinical history, physical examination, and the usual laboratory studies, a clear decision in the detection of early AA is lacking. Ultrasonography has been used increasingly in the past years, with positive results and high sensitivity and specificity rates [6]. In skilled hands, ultrasonography has proven to be an effective diagnostic tool. A prospective study showed that ultrasonography was more accurate than the surgeon’s initial clinical impression in AA diagnosis [7].
One of the key questions is to what extant laboratory tests are helpful in the early diagnosis of AA in children. For a long time, the main laboratory test has been the leucocyte count. The diagnostic value of laboratory inflammatory markers has been studied in the past years with different and contradictory results [8].
In recent years, some authors reported that the neutrophil-to-lymphocyte ratio (NLR) is a predictor of inflammation and a useful marker in the preoperative diagnosis of AA [9–11]. Kahramanca et al. [12] suggested that NLR calculation may provide a sensitive parameter in the preoperative prediction of AA and prevent negative appendectomy based on its predictive value. Higher specificity and sensitivity have been reported using an NLR greater than 3.5 [5]. Furthermore, some authors reported that the NLR may help in distinguishing complicated from non-complicated appendicitis. However, there are studies in which this observation was not confirmed [13, 14].
Results of the studies conducted thus far have shown that NLR could serve as a simple parameter in the assessment of inflammatory status in adults [15]. Studies investigating the use of NLR in children are scarce, and to the best of our knowledge, NLR was not investigated as a possible marker of AA in Bosnian children. Thus, the aim of present study was to analyse the contribution of NLR in the diagnosis and prediction of AA complications in Bosnian children.
Material and methods
The descriptive cross-sectional study included 170 patients, of both genders, under the age of 15 years, admitted for acute abdominal pain in the Clinic of Paediatric Surgery, University Clinical Centre Sarajevo (UCCS), between October 1st, 2016 and March 30th, 2017.
The inclusion criteria were the presence of some of the leading symptoms indicating the existence of AA: abdominal pain localised in the lower right quadrant, nausea, vomiting, loss of appetite, dry tongue, constipation, elevated body temperature, poor general condition, and tachycardia. The exclusion criteria were: patients aged > 15 years; patients with acute disease of gastrointestinal tract (biliary colic, intestinal obstruction, pancreatitis); patients with inborn congenital malformation of gastrointestinal tract; patients with acute urological disorders and acute urinary tract infections (renal colic, pyelonephritis); female patients with inflammation and ovarian disease; patients with respiratory tract infections with abdominal pain; children with disabilities; children with abdominal injury; and appendectomies patients and patients with chronic abdominal pain.
The study protocol was approved by the Ethical Committee of the UCCS, registered under number 0302-20248. All participants signed informed, written consent after the explanation of the study procedure. All procedures were conducted in an accordance with the guidelines of the World Medical Association Declaration of Helsinki for human subjects.
All subjects went through a detailed anamnestic questionnaire, physical examination, measurement of body mass index (BMI) and body temperature, Alvarado score assessment, ultrasonography (US), and standard laboratory analyses. On admission, all children were examined by an experienced paediatric surgeon, and according to the clinical judgment, the patients were classified to those to be operated or those to be left under observation.
Non-operated patients with suspected AA, who were left under observation constituted Group 1 (n = 94), while patients who underwent appendectomy constituted Group 2 (n = 76). Based on the histological findings of the removed appendix patients of Group 2 were subdivided into three groups: Group A – patients with phlegmonous AA; Group B – patients with gangrenous AA; and Group C – patients with perforated AA [2].
Blood samples were taken from the cubital vein using vacutainer technique. The white blood cell count (WBC), and neutrophil and lymphocyte count were measured on an automatic haematology analyser (CELL-DYN Ruby; Abbott Laboratories, USA) at the Department of Biochemistry, UCCS, Sarajevo. The NLR value was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
High sensitivity C-reactive protein (CRP) was determined by particle-enhanced immunonephelometry (BN System, Dade Behring, Marburg, Germany) at the Department of Biochemistry, UCCS, Sarajevo.
Statistical analysis
The Kolmogorov-Smirnov test of normality was used to test the distribution of variables. Because all variables were skewed they were presented as medians and interquartile ranges. Categorical variables were shown as frequencies. The difference in values of parameters that showed skewed distribution was assessed by Mann-Whitney U test and Kruskal-Wallis test. Chi-square analysis was used for categorical variables. In order to determinate the factors associated with NLR, multivariate regression analysis was performed. To determine optimal cut-off values of NLR for differentiation between non-operated patients with suspected AA and patients who underwent appendectomy, as well as differentiation of patients with different pathologic grades of AA, receiver operating characteristic (ROC) curves and their corresponding areas under the curve (AUC) were used. The accuracy rate for ROC curves was calculated with 95% confidence interval (95% CI). A p value < 0.05 was considered statistically significant for all comparisons. The software used was SPSS for Windows (version 17.0; SPSS, Chicago, IL, USA).
Results
The clinical and laboratory characteristics of Group 1 and Group 2 are presented in Table I. There were no statistically significant intergroup differences in age, sex, BMI, and body temperature (≤ 37/> 37°C). A statistically significant difference (p < 0.001) was observed in ultrasonography and Alvarado score between Group 1 and Group 2. Laboratory findings showed a significant difference in median NLR value (p < 0.001), WBC count (p < 0.001), neutrophil count (p < 0.001), lymphocyte count (p = 0.018), and CRP values (p < 0.001) when Group 1 was compared with Group 2.
Table I
Clinical and laboratory parameters in Group 1 and in Group 2 patients
Table II shows the clinical and laboratory characteristics of patients with different pathologic grades of AA. Subjects did not differ in the following: age, sex, and BMI. A statistically significant difference (p = 0.001) was observed in neutrophil count, NLR value, and Alvarado score between Group A and Group B. The patients in Group C had significantly higher WBC count, neutrophil count, NLR value, Alvarado score, and CRP value compared to patients in Group A (p < 0.001). We also observed a significant difference (p < 0.01) in WBC count, CRP value, and ultrasonography when patients in Group A were compared to patients in Group B, as well as, in lymphocyte count between patients in Group A and patients in Group C. The patients in Group C had significantly higher (p < 0.05) WBC count and CRP values in comparison with patients in Group B. Ultrasonography and body temperature (≤ 37/> 37°C) were statistically significantly different (p < 0.05) when patients in Group C were compared with patients in Group A.
Table II
Clinical and laboratory parameters of patients with different pathologic grades of AA
| Variable | Group A (n = 31) | Group B (n = 20) | Group C (n = 25) |
|---|---|---|---|
| Age [years] | 11.0 (7.0–12.5) | 11.8 (7.6–13.9) | 8.5 (7.0–12.0) |
| Sex (male/female) | 18/13 | 13/7 | 11/14 |
| US (negative/positive) | 9/22 | 0/20♦ | 2/23 |
| Body temperature (≤ 37C/> 37°C) | 22/9 | 11/9 | 10/15 |
| BMI [kg/m2] | 18.1 (17.1–20.6) | 18.9 (17.7–19.5) | 17.0 (15.5–20.4) |
| Alvarado score | 4 (3–6) | 7 (6–9)* | 8 (7–9)# |
| WBC count [× 103/mm3] | 9.0 (7.0–11.8) | 15.8 (11.2–18.2)♦♠ | 18.5 (14.8–3.8)# |
| Neutrophil count [× 103/mm3] | 4.9 (3.3–8.3) | 11.5 (8.5–15.3)* | 14.6 (9.9–20.6)# |
| Lymphocyte count [× 103/mm3] | 2.4 (1.5–3.4) | 1.7 (1.2–2.5) | 1.6 (1.2–2.2)♦ |
| CRP [mg/dl] | 7.6 (0.8–24.7) | 29.2 (9.9–42.0)♦♠ | 54.7 (20.2–84.3)# |
| NLR | 1.84 (1.2–4.9) | 6.5 (2.8–9.9)* | 9.6 (5.2–13.1)# |
Median (25th and 75th percentiles); X2 test; Kruskal-Wallis test. BMI – body mass index, US – ultrasonography, WBC – white blood cell, CRP – C-reactive protein, NLR – neutrophil-to-lymphocyte ratio. Group A – patients with phlegmonous AA, Group B – patients with gangrenous AA, Group C– patients with perforated AA.
We further performed ROC analysis to investigate the capacity of the NLR value in differentiating patients in Group 1 from patients in Group 2, as well as in differentiating patients with different pathologic grades of AA.
Optimal cut-off value of NLR determined by ROC curve in differentiating patients in Group 1 vs. patients in Group 2 was ≥ 3.48. AUC for determined cut-off value was 0.693 with 95% CI of 0.612–0.774 (p < 0.001). For a calculated optimal NLR cut-off value of ≥ 3.48, the maximal specificity was 74% and maximal sensitivity 63% (Figure 1A).
Figure 1
A – Optimal cut-off value of NLR value determined by ROC curve for distinguishing between patients in Group 1 and patients in Group 2. B – Optimal cut-off value of NLR value determined by ROC curve for distinguishing between patients in Group A and patients in Group C. C – Optimal cut-off value of NLR value determined by ROC curve for distinguishing between patients in Group A and patients in Group B
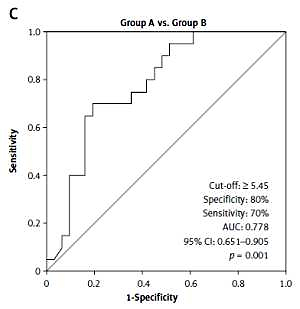
Optimal cut-off value of NLR determined by ROC curve in differentiating patients in Group A from patients in Group C was ≥ 5.61. The AUC for a determined cut-off value was 0.840 with a 95% CI of 0.729–0.951 (p < 0.001). For a calculated optimal NLR cut-off value of ≥ 5.61, the maximal specificity was 81% and maximal sensitivity was 79% (Figure 1B).
Optimal cut-off value of NLR determined by ROC curve in differentiating patients in Group A from patients in Group B was ≥ 5.45. The AUC for a determined cut-off value was 0.778 with a 95%CI of 0.651–0.905 (p = 0.001). For a calculated optimal NLR cut-off value of ≥ 5.45, the maximal specificity was 80% and maximal sensitivity 70% (Figure 1C).
Results of multiple regression analysis revealed that none of the studied laboratory parameters was associated with the NLR value as a dependent variable in Group 1. In Group 2 multiple regression analysis showed that CRP values, lymphocyte count, and neutrophil count were associated with NLR (Table III).
Table III
Multiple regression analysis for neutrophil-to-lymphocyte ratio as a dependent variable in Group 2
Subsequently, we conducted multivariate analysis for the outcomes (severity of AA). Results have shown that only CRP of all of the tested variables was associated with the NLR in Group A and Group C but not in Group B (Table IV).
Table IV
Multiple regression analysis for neutrophil-to-lymphocyte ratio as a dependent variable in patients with different pathologic grades of acute appendicitis
| Independent variables | Group A OR (95% CI) | Group B OR (95% CI) | Group C OR (95% CI) |
|---|---|---|---|
| WBC count [× 103/mm3] | –0.106 (–1.376 to 1.164) | –1.008 (–3.797 to 1.782) | 0.024 (–0.801 to 0.849) |
| Neutrophil count [× 103/mm3] | 0.705 (–0.617 to 2.027) | 1.717 (–1.358 to 4.792) | 0.521 (–0.309 to 1.352) |
| Lymphocyte count [× 103/mm3] | –1.303 (–2.774 to 0.168) | –1.054 (–4.874 to 2.765) | –1.029 (–2.589 to 0.530) |
| CRP [mg/dl] | –0.031* (–0.056 to –0.007) | –0.013 (–0.073 to 0.046) | 0.058# (0.031 to 0.085) |
Furthermore, in an attempt to assess whether NLR has greater prognostic value than CRP in diagnosis and in differentiating patients with different forms of AA complications, we performed ROC analysis. The results showed that NLR has greater prognostic value between all groups except between Group B and Group C (data not shown).
Discussion
Early diagnosis of AA is not always easy. Acute appendicitis as a relatively harmless disease, but it often turns into a serious condition that is life threatening, because perforation takes place in 1/3 of patients before treatment. A large number of postoperative complications also indicates the seriousness of the problem. The number of misdiagnoses is significant, resulting in a large number of children who are unnecessarily exposed to the risk of operation. At the same time, many children are surgically treated relatively late, after perforation, which increases the number of postoperative complications. In order to reduce the rate of complications and negative appendectomy in patients with AA, it is necessary to establish the diagnosis earlier. Unfortunately, there is no laboratory marker for the accurate and certain diagnosis of AA [12].
NLR is a marker of systemic inflammatory response, and it can be easily obtained from differential WBC count [16]. Previous studies reported that NLR is a significant prognostic factor in patients with coronary artery disease [17], various malignancies [18, 19], and gastrointestinal stromal tumour [16]. Ozcicek et al. [20] demonstrated that NLR may serve as independent predictor of epicardial adipose tissue in haemodialysis patients. Furthermore, the results of Soylu et al. [21] showed that in patients presenting with acute pulmonary embolism, the NLR value was an independent predictor of in-hospital mortality. The predictive value of NLR in the diagnosis and prediction of AA complications in children has not been studied sufficiently.
The results of our study showed that NLR value was significantly higher (p < 0.001) in Group 2 compared with the NLR level in Group 1. Our results are in the accordance with the results of Yazici et al. [9], who found in children statistically significant differences in NLR values between appendicitis patients who were treated operatively compared with patients with non-specific abdominal pain. Sevim et al. [22] also observed significantly higher NLR levels in adult patients with AA compared to patients without AA. Significantly higher NLR level in adult patients with positive appendectomy compared to patients with negative appendectomy is also supported by the findings of Kahramanca et al. [12]. The statistically significantly lower NLR values in Group 1, observed in our and in previous studies, suggest that NLR value may be helpful in differentiating non-operated vs. operated patients with AA [9, 12, 22].
The results of our study showed that the NLR value was significantly different between patients with different pathologic grades of AA (p < 0.001). Namely, the NLR value was significantly lower in Group A compared to patients in Group B (p = 0.001) and patients in Group C (p < 0.001). Comparing the NLR values between patients in Group B and patients in Group C, we found no significant difference (p = 0.09).
The obtained results are consistent with the results of Kahramanca et al. [12], who found significantly higher NLR values in adult patients with complicated appendicitis (gangrenous and perforated) than in patients with non-complicated appendicitis. Our results confirm findings from previous studies such as the study by Ishizuka et al. [23] and Shimizu et al. [24], who also observed significant difference of NLR values between patients with different pathological type of AA: catarrhal, phlegmonous, and gangrenous appendicitis. Conversely, the results of our study are not completely in accordance with the results of Kaykisiz et al. [13] and Akgül et al. [14], who reported significant differences in NLR value only between non-appendicitis patients and AA patients. When they compared uncomplicated AA with complicated AA, they did not find statistically significant differences in NLR values.
In our study, the optimal NLR cut-off value in distinguishing between patients in Group 1 and patients in Group 2 was ≥ 3.48 and showed a moderate sensitivity (63%) and good specificity (74%). Our results are in agreement with the findings of Sevim et al. [22], who found an optimal NLR cut-off value of 3.5 and good sensitivity (76.6%) and low specificity (59.3%) in distinguishing patients with and without AA. Bialas et al. [25] investigated the usefulness of the NLR value in the diagnosis of appendicitis in the adult population. Their retrospective study included 469 patients operated for AA. They determined an NLR cut-off value of 3.5, similarly to our results, but the sensitivity (77.5%) was higher and specificity (73.3%) was lower than in our study. The authors concluded that due to the ease of calculation and high rate of false positive and negative diagnosis, the NLR value is a possible marker in establishing a diagnosis of appendicitis. Kahramanca et al. [12] investigated the ability of NLR to predict AA preoperatively and to differentiate between patients with and without AA. Based on the determined optimal cut-off value by ROC curve analysis, NLR values showed a moderate sensitivity (65.3%) and low specificity (54.7%) in distinguishing patients with and without AA, and good sensitivity (70.8%) and low specificity (48.5%) in distinguishing complicated from non-complicated appendicitis. These authors suggest that preoperative NLR is a useful parameter in diagnosis of AA and in differentiation between simple and complicate appendicitis.
In addition, the results of our study showed that none of the studied laboratory parameters were associated with the NLR in Group 1. However, in Group 2 multiple regression analysis demonstrated that CRP values, lymphocyte count, and neutrophil count were closely associated with NLR. The results of univariate analysis reported by Ishizuka et al. [23] revealed that age, sex, fever, CRP, albumin, Glasgow Prognostic Score, and NLR were associated with gangrenous appendicitis. However, multivariate analysis disclosed that only age and NLR were associated with gangrenous appendicitis. In an attempt to assess whether NLR has greater prognostic value than CRP in the diagnosis and in differentiating patients with different forms of AA complications, we performed ROC analysis. The results showed that NLR has greater prognostic value between all groups except between Group B and Group C, suggesting that NLR is a better marker in the diagnosis of AA and in differentiating patients with different grade of AA complications than CRP.
In interpreting the findings of the current study, several limitations should be acknowledged. Firstly, the sample size was small, consisting of children with acute abdominal pain suspected of having an AA from a select population and, therefore, the results cannot be generalised over the whole population. Secondly, the cross-sectional design of the study prevents us from deducing any causal relations between our findings. However, the results of our study suggest that NLR could be used as a simple, non-invasive, reliable, and readily available test in the diagnosis and prediction of AA complications in children.
In conclusion, the accurate and timely diagnosis of AA is very important because it reduces the number of negative appendectomies. The results of our study showed that NLR may serve as a marker in differentiating non-operated patients with suspected AA from patients who underwent appendectomy. Moreover, the obtained findings suggest that in distinguishing patients with different pathologic grades of AA, NLR had good diagnostic accuracy. To draw definite conclusions on the predictive power of NLR as a marker of AA large multicentric studies are required.