Introduction
The disease COVID-19, which is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), has had large scale ramifications as a global pandemic [1, 2]. The virus was first detected in Wuhan, China in December 2019, and has since progressively spread worldwide and infected more than 91 million people from numerous countries [3–5]. Emerging data have suggested a generally comparable disease course in pregnant and non-pregnant women [6–8]. However, the physiological changes accompanying pregnancy (such as decreased functional residual volume, increased stroke volume, and edema of the respiratory tract mucosa) [9, 10] and the possibility of adverse neonatal complications necessitate vigilant efforts to thoroughly assess possible risk factors for poor SARS-CoV-2 outcome in the pregnant population.
Previous reports have associated an increased risk of perinatal mortality and preterm birth in pregnant women diagnosed with viral pneumonia [11–13]. In 2004, SARS-CoV-1 was linked to an increase rate of maternal morbidity and mortality, intrauterine growth restriction, miscarriage and preterm delivery amongst infected mothers [14, 15]. However, it is not yet clear how SARS-CoV-2 may influence pregnancy and the risk factors it presents to the mother and unborn child [16]. More precisely, due to the novelty of the virus and relatively recent spread, there are extremely scarce data on maternal and perinatal outcomes when the infection is contracted in the first or second trimester of pregnancy. The most recent report by the CDC placed emphasis on COVID-19 adverse effects on pregnancy and urged the need for more extensive investigation in this field [17]. Assessing the relationship of COVID-19 with the early gestational trimesters of pregnancy is key to effective counseling, and offers invaluable insight on how the pandemic may influence pregnancy at large in the foreseeable future.
Material and methods
Search strategy and selection
The present review paper was guided by established protocols for healthcare systematic reviews [18]. A systematic search was conducted by two independent reviewers, using the primary databases PubMed and ScienceDirect. The search included articles published from January 1 to December 27, 2020. A secondary database, Google Scholar, was also used to provide broader coverage of published literature. The keywords “COVID-19” OR “SARS-CoV-2” were followed by each of “pregnancy” OR “gestation” OR “maternal” during the search. Reference lists of high value studies were also screened for potential relevant studies.
Reports included in this review consisted of case-report, cross-sectional, case-control, cohort (both prospective and retrospective) and case series. Studies that reported pregnant women, who contracted COVID-19 in either their first (1–12 weeks of gestation) or second trimester (13–28 weeks gestation), were of main interest. Duplicate studies, unrelated topics, reviews, guidelines, surveys, non-English language studies, and studies that included COVID-19 positive mothers contracting the virus in the third trimester were all excluded based on view of the abstract and paper. Any studies with unclear distinction on time of COVID-19 contraction during pregnancy were, after thorough assessment, not considered for results.
Retrieving relevant studies and data extraction
Following the initial search to identify possible relevant studies, the two reviewers excluded all duplicates. Next, the studies were briefly screened for their overall relatedness to the topic and excluded if deemed not valuable to the systematic review. A list was compiled of the studies with potential relevant data, and the two reviewers independently and thoroughly assessed them for eligibility. Following this process, a final list of relevant studies was compiled. Consensus between reviewers was reached concerning the final studies included.
Having established the group of studies that comprise the systematic review, data were extracted by the two reviewers independently into a standard form. The form included structured subheadings of the clinical data of interest, such as: symptoms, pregnancy-related complications, laboratory and radiological findings, maternal and neonatal outcomes (stillbirth (death after 20 weeks of gestation), miscarriage (death before 20 weeks of gestation), terminated, delivered/alive, or ongoing pregnancy), and others (drugs administered, placental changes). The data were cross-checked for any inaccuracies by a third reviewer. A high level of scrutiny was applied as the data extracted strictly concerned 1st and 2nd trimester contraction of COVID-19 in pregnant women. Any data that were merged with 3rd trimester mothers or did not clearly attribute the data to the trimesters of interest were excluded. There was a discussion and a consensus was reached between all reviewers as to which variables to include in the qualitative synthesis.
To give an estimate of overall incidence, a combined incidence rate of certain clinical phenomena (symptoms, comorbidities, complications, etc.) was calculated by adding the individual incidences in the respective studies and constructing a total percentage. Only studies with contributing data were included in the calculation. The constructed values should be interpreted with caution.
Quality assessment
A combination of the STROBE statement (Strengthening the Reporting of Observational Studies in Epidemiology) [19] and CERQual approach [20] was implemented in quality assessment of studies and findings. Studies included in the systematic review were individually evaluated using the STROBE statement, with careful appraisal of methodological limitations, studied population, epidemiological information (such as mode of transmission), follow-up information, selection and other bias, as well as incomplete reporting of data.
Providing a transparent explanation, and following the CERQual assessment outline, the review findings were then allocated a confidence score. The confidence score is based on the combined quality of studies contributing to the finding, with particular emphasis on adequacy of data, methodological limitations, consistency in findings or coherence, and relevance of article to review topic. Confidence was assigned using one of the four statements: very low, low, moderate, and high (“unclear”, “it is possible”, “likely”, and “very likely” – that the review finding is a reasonable representation of phenomena, respectively). The constructed review follows the standardized PRISMA guidelines to allow for elaborate presentation [21].
Results
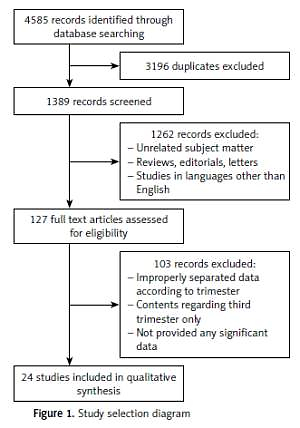
The search revealed 4585 findings, of which 3196 were noted as duplicates. Of the total 1389 articles, 1262 studies were excluded based on screening of the title, abstract, and brief view of the paper. The majority of exclusions were based on unrelatedness to subject matter or study type (reviews, guidelines). Full text assessment of the remaining 127 articles yielded a total of 24 studies that met the inclusion criteria and were considered relevant to the review (Figure 1). One hundred and three records were removed largely due to an improper separation of data between 1st/2nd and 3rd trimester COVID-19 patients, with the majority reporting combined results. A combined total of 220 first and second trimester pregnant women with COVID-19 were included.
Study characteristics
The majority of the studies were published between March and December 2020 and they originated from 11 different countries. Maternal age range was 21–49 years. The range of gestation when the mothers contracted the virus was 5–27 weeks. Most patients had COVID-19 diagnosis confirmed by positive RT-PCR. At the time of publication, 10 studies reported ongoing pregnancies; of the remaining 14 studies, 3 reported neonatal survival while rest had unclear status (Table I). In one particular study reported through the CDC, stillborn or fetal death (at < 20 weeks) was observed in 4 infants; the outcomes of the remaining neonates remained unspecified [17]. Study characteristics and clinical features can be found in Table I.
Table I
Symptoms, complications, laboratory and radiological findings, neonatal outcomes
| Authors | Country | Pub. date | No. of patients (trimester) | Symptoms | Pregnancy-related complications | Laboratory findings | Radiological findings | Neonatal outcomes |
|---|---|---|---|---|---|---|---|---|
| Delahoy et al. [17] | USA | Sep 2020 | 14 (1st trim) 61 (2nd trim) | Fever, cough, myalgia, SOB | N/A | N/A | N/A | 4: miscarriage (1st trim) |
| Hantoushzadeh et al. [22] | Iran | July 2020 | 3 (2nd trim) | Fever, cough, dyspnea, tachycardia | ARDS, pneumothorax, ARF, cardiopulmonary collapse, septic shock, DIC, left HF | N/A | Patchy ground-glass feature on CT | 2 (twins): IUFD 2 (twins): premature labor; death at day 3 1: stillbirth |
| Browne et al. [23] | USA | June 2020 | 1 (2nd trim) | Myalgia, fever, cough | Hyperemesis gravidarum, acid reflux | Elevated WBC, low HCT | N/A | Ongoing twin preg |
| Baud et al. [24] | Switzerland | June 2020 | 1 (2nd trim) | Fever, cough, myalgia, fatigue | Funisitis | N/A | N/A | Stillbirth |
| Richtmann et al. [25] | Brazil | July 2020 | 3 (2nd trim) | Fever, cough, myalgia | Chorioamnionitis | Normal | N/A | 3: stillbirth |
| Hosier et al. [26] | USA | June 2020 | 1 (2nd trim) | Fever, cough, myalgia, fatigue, diarrhea | Preeclampsia, DIC | Elevated ALT, AST, urine protein, prolonged PTT, decreased fibrinogen | Hazy opacity | Opted for termination of preg |
| Garcia-Manau et al. [27] | Spain | July 2020 | 2 (2nd trim) | “Mild symptoms” | DIC, preeclampsia, Fetal skin edema on ultrasound | N/A | N/A | Ongoing preg |
| Lokken et al. [28] | USA | Aug 2020 | 3 (2nd trim) | Dyspnea | N/A | Normal | Consolidation | Ongoing preg |
| Wu et al. [29] | China | Aug 2020 | 5 (1st trim) 2 (2nd trim) | Fever, cough, myalgia | N/A | Elevated ALT, AST and CRP, lymphocytopenia | Pneumonia on CT | 1: chemical preg (1st trim) 6: ongoing preg |
| Yu et al. [30] | China | April 2020 | 2 (1st trim) | Fever, diarrhea, cough, dyspnea | N/A | N/A | Pneumonia on CT | Ongoing preg |
| Liu et al. [31] | China | March 2020 | 2 (2nd trim) | Fever, fatigue, dyspnea, | N/A | N/A | N/A | Both neonates survived |
| Wang et al. [32] | China | July 2020 | 1 (2nd trim) | Myalgia, fever, cough, dyspnea | N/A | Decreased Hb content and serum albumin, elevated neutrophil ratio and ALT, AST | Consolidation | Ongoing preg |
| Panichaya et al. [33] | Thailand | July 2020 | 1 (2nd trim) | Fever, chest discomfort | N/A | Normal | Normal | Opted for termination of preg |
| Inchingolo et al. [34] | Italy | July 2020 | 1 (2nd trim) | Fever, cough | N/A | N/A | Pneumonia | N/A |
| Crovetto et al. [35] | Spain | Aug 2020 | 54 (1st trim) | Fever, cough, diarrhea, loss of taste or smell | N/A | N/A | N/A | N/A |
| Hachem et al. [36] | France | July 2020 | 1 (2nd trim) | Normal | N/A | Elevated CRP, lymphocytopenia | Pneumonia on CTPA | Miscarriage |
| Piersigilli et al. [37] | Belgium | June 2020 | 1 (2nd trim) | Normal | Cholecystitis, HELLP syndrome | Elevated CRP | Pneumonia on X-ray | Neonate survived |
| Cosma et al. [38] | Italy | July 2020 | 14 (1st trim) | Fever, diarrhea, cough, loss taste/smell, arthralgia | N/A | N/A | N/A | N/A |
| Lamazou et al. [39] | France | May 2020 | 1 (1st trim) | Dyspnea, nausea, asthenia | Liver (hepatic) cytolysis | N/A | N/A | Ongoing preg |
| Tang et al. [40] | China | July 2020 | 2 (2nd trim) | Fever, dyspnea | Gestational diabetes, neonatal jaundice | Elevated CRP, lymphocytopenia | Ground glass opacity on CT | Neonates survived |
| Hong et al. [41] | USA | July 2020 | 1 (2nd trim) | Fever, cough, dyspnea, myalgia | N/A | N/A | Normal | Ongoing preg |
| Rubio Lorente et al. [42] | Spain | Aug 2020 | 1 (1st trim) 1 (2nd trim) | Fever, rhinorrhea, cough | N/A | N/A | N/A | 1: ongoing preg 1: abortion (1st trim) |
| la Cour Freiesleben et al. [43] | Denmark | Dec 2020 | 18 (1st trim) | Loss of taste or smell, dry cough, fatigue, fever | N/A | N/A | N/A | 1 fetal death 17 ongoing preg |
| Cosma et al. [44] | Italy | Oct 2020 | 23 (1st trim) | Fever, cough, arthralgia | N/A | N/A | N/A | N/A |
[i] Trim – trimester, DIC – disseminated intravascular coagulation, Hb – hemoglobin, ALT – alanine aminotransaminase, AST – aspartate aminotransferase, CRP – C-reactive protein, PTT – partial thromboplastin time, Preg – pregnancy, N/A – not available, HCT – hematocrit, CTPA – CT pulmonary angiogram, ARF – acute renal failure, HF – heart failure, IUFD – intrauterine fetal demise, SOB – shortness of breath.
Symptoms
All studies [17, 22–44] disclosed symptom presentation, pertaining to 220 pregnant women. Despite a majority of women not reporting any symptoms, the most frequently observed symptoms and clinical signs were fever (86/220 = 39%) and cough (73/220 = 33%). To a smaller extent, dyspnea (52/220 = 24%), nausea and vomiting (27/220 = 12%), and loss of taste or smell (57/220 = 26%) were observed. Out of the 220 first and second trimester pregnant women included in this review, 132 were symptomatic (60%).
Laboratory and radiological findings
Radiological findings of 24 pregnant women in 12 studies were reported [22, 26, 28–30, 32–34, 36, 37, 40, 41]. Pneumonia and ground glass appearance were the first (n = 12/24: 50%) and second (n = 5/24: 21%) most common observed radiological findings respectively. More than half of the reported findings revealed bilateral distribution of the disease. Normal radiological findings were also noted in 2/24 patients. The laboratory results of 21 pregnant women were reported. Elevated C-reactive protein as well as lymphocytopenia were observed in 4/21 women (19%), and high levels of hepatic markers (AST/ALT) were recorded in 9 patients (43%) (Table I).
Pregnancy-related complications
Only a portion of the studies (9) reported some sort of complication during the span of pregnancy which pertained to 15 patients [22–27, 37, 39, 40]. Disseminated intravascular coagulation (DIC) (20%; 3/15), preeclampsia (13%; 2/15), as well as liver cytolysis, chorioamnionitis, ARDS, hyperemesis gravidarum, pneumothorax and gestational diabetes (each observed in 1/15 of the reported patients) were the most commonly observed complications.
Maternal outcomes
The overwhelming majority of mothers included in this review were successfully discharged from the hospital or were alive as of the date of publication (220 women). However, Hantoushzadeh et al. reported 3 cases of which COVID-19 maternal death was the outcome [22]. No other significant maternal outcomes were reported.
Neonatal outcomes
Out of 223 neonates, the outcomes of 60 infants (31/60: maternal infection during 1st trimester and 29/60: 2nd trimester infections) from 20 studies were specified as deceased/terminated, miscarriage, alive/delivered, or still ongoing pregnancy [17, 22–33, 36, 37, 39–43]. While the majority of 1st trimester infection neonates had a reported status of ongoing pregnancy (n = 24/31: 77.4%), 4/31 (12.9%) reported miscarriages, 1/31 (3.2%) abortion, 1 (3.2%) stillbirth and 1 (3.2%) chemical pregnancy were also noted.
In the studies with documented maternal COVID-19 infection during the second trimester of pregnancy, neonatal outcomes were as follows: 5/29 (17.2%) completed term and were delivered healthy, 14/29 (48.2%) had ongoing pregnancy, 5/29 (17.2%) stillbirths. 1/29 (3.4%) miscarriage, 2/29 (6.9%) intrauterine fetal demise (IUFD), and 2/29 (6.9%) opted for termination of pregnancy. The rest of the neonates (n = 163/223 (73.1%)) had unreported status concerning the outcome of pregnancy (unclear whether delivered, deceased, or ongoing pregnancy).
Others
A minority of studies [24–26, 33, 42] documented the condition of 7 placentas upon delivery, of which 2/7 were normal. Histopathological findings documented were: acute villitis and intervillitis (2/7 (28.6%)), acute deciduitis (1/7 (14.3%)), diffuse perivillous fibrin (5/7 (71.4%)) and an inflammatory infiltrate composed of either neutrophils, T-lymphocytes or macrophages (2/7 (28.6%)). One of the placental histopathological investigations determined no membrane inflammation or viral inclusion [33]. A case of decidual inflammation was also reported, but was eventually declared to be caused by abortion of the first trimester rather than COVID-19 disease [42].
Drug treatment of 12 patients in 9 studies was reported [22–27, 31, 36, 41]. Azithromycin (5/12 = 42%) and hydroxychloroquine (5/12 = 42%) were the most commonly administered medications. Other medications such as lopinavir-ritonavir (4/12 = 33%), oseltamivir (3/12 = 33%), acetaminophen (2/12 = 17%) and ceftriaxone (2/12 = 17%) were also reported.
Qualitative synthesis
The majority of included studies were retrospective. Epidemiological information and mode of contraction were only recorded in 7 of the studies [23, 29, 30, 32, 33, 36, 40]. The virus was usually transmitted through direct contact with an exposed spouse. Mainly, studies included only hospitalized or severe cases, which was noted as a minority of cases in the respective article, and as such was considered as selection bias [22, 25, 26, 28, 30–33, 35, 36]. Other methodological limitations include short follow-up, with a majority being less than 1 month. Nine studies were of women with still ongoing pregnancy [23, 27–30, 32, 39, 41, 42], and only 3 studies had recorded the complete term of pregnancy [31, 37, 40]. Pregnancy-related complications were under-reported, with 9/24 studies mentioning incidence of any complications [22–27, 37, 39, 40]. Overall limited data were available, as 15 studies reported ≤ 2 patients [23, 24, 26, 27, 30–34, 36, 37, 39–42]. Lack of extensive data, short follow-up, and a large proportion of ongoing pregnancies resulted in no findings receiving a “high” confidence score. CERQual confidence in review findings and their respective explanation can be found in Table II.
Table II
CERQual assessment of review findings
Discussion
Despite the majority of symptoms presenting as mild, pregnant women and newborns are a worrisome population considering that there are very limited data in regards to the outcome and complications in COVID-19 infected pregnant women [45]. During the SARS-CoV-1 outbreak in 2002–2003, a high maternal mortality rate was observed [14, 46]. Infected pregnant mothers who survived reported experiencing 1st trimester miscarriages and intrauterine growth restriction (IUGR) during their 2nd and 3rd trimesters [14]. Considering the similarities, including genetic makeup and mode of cell entry, between SARS-CoV-1 and the recent SARS-CoV-2 [47], particular attention should be placed on the pregnant population in order to identify and attend to any possible detrimental complications.
To the best of the author’s knowledge, this is the first qualitative systematic review focused on the clinical characteristics of 1st/2nd trimester infections of SARS-CoV-2 during pregnancies. Primary data showed high coherence in relation to symptoms, reporting fever (39%) and cough (33%) to be the most prevalent in the population group of 1st and 2nd trimester pregnant women. A few other studies have reported similar results [48, 49]. Compared to 3rd trimester infections with SARS-CoV-2, comparable results have been reported in terms of symptoms. In one study that included 108 SARS-CoV-2 positive pregnant women during their third trimester, the majority of the patients experienced fever (68%), while cough was observed in only 34% of the patients [50]. Elevated body temperature during pregnancy is associated with disruption of cell migration, damage to the vascular system and death of neuroblast cells [51]. Hence, this is a potential concern during the organogenesis period that takes place during the 1st trimester of pregnancy [52]. Nevertheless, further research is required to analyze the association of certain symptoms with maternal and neonatal outcomes.
According to our systematic review, 1st and 2nd trimester pregnant women could potentially be at risk of experiencing placental inflammation such as villitis/intervillitis and perivillous fibrin deposition. The literature defines villitis as a damaging inflammation that is characterized by maternal T-cell invasion into chorionic villi [53]. Furthermore, an increase in proinflammatory markers such as tumor necrosis factor (TNF-α) and interleukin (IL)-6 is characteristic of COVID-19 infection and has been associated with endothelial activation and apoptosis of the trophoblastic cells [54]. It is unclear whether these placental findings can be attributed to the severity of the infection when contracted during the 1st and 2nd trimester or perhaps be linked to the overall outcome of the neonates’ well-being. It is worth noting that 4 of the 17 (23%) recorded neonatal deaths were of pregnancies with developed inflammation such as intervillitis/villitis and perivillous fibrin deposition of the placenta. In some third trimesters delivered placentas, highly abnormal findings such as extensive intervillositis as well as perivillous fibrin were recorded on histopathological examination [55, 56]. Similarly, in a study regarding cases of SARS-CoV-1, three placentas were delivered from pregnancies in which the mothers had acute infection – these were abnormal and demonstrated increased subchorionic and intervillous fibrin [57]. Such findings can be associated with abnormal maternal blood flow to the placenta. Overall, placental histological findings were under-reported in the studies included in this review. This raises concerns for inadequacy of the data and highlights the need for larger samples.
The radiological results of the studies assessed were largely definitive for CT diagnosed pneumonia. Pneumonia features, such as consolidations and ground glass opacities, were the most commonly reported changes observed in roughly half of the patients and one-fifth of the patients, respectively. The majority of the reports indicate a bilateral spread of the disease. In pregnant women infected with COVID-19 during their 3rd trimester, similar patterns of consolidations and patchy ground glass opacities were observed on the CT scans [58]. These radiological changes appear to be the pattern for COVID-19 cases in general, including that of older aged individuals [59]. Chest CT plays a vital role in the initial assessment, treatment and follow-up of COVID-19 patients, and some studies have even shown that chest CT sensitivity could be preferable to RT-PCR for early identification of COVID-19 [60, 61].
In the small portion of studies that detailed therapeutic treatments for the COVID-19 infected pregnant women, azithromycin (42%) and hydroxychloroquine (42%) were the most common, followed by lopinavir-ritonavir (33%) and oseltamivir (33%). The inconsistency in the administration of drugs underlies the lack of sufficient data for therapeutic efficacy against the novel coronavirus. A study concerning the safety of hydroxychloroquine in 1st trimester pregnancies reported no notable changes in the nervous/cardiac system formation, prematurity or birth weight of the infants [62]. Despite some data indicating the efficacy of hydroxychloroquine against SARS-CoV-2 [63], consistent clinical results are yet to be presented. Furthermore, lopinavir and ritonavir have also shown an inconsequential effect on pregnancy, and in their study of 955 pregnant women, Roberts et al. observed that the incidence of birth defects in children with prenatal exposure to lopinavir/ritonavir did not differ substantially from the control [64]. Hence, similar to hydroxychloroquine, lopinavir and ritonavir are being used as possible deterrents for COVID-19 exacerbation in pregnant women. Further studies are necessary to examine the curative role of pharmaceutical drugs in the context of COVID-19 during pregnancy, and may allow for more consistent and effective treatment plans.
The reported complications associated with COVID-19 pregnancy may influence the adverse outcomes of first and second trimester pregnant women and their newborns. 10 of our neonatal deaths concerned mothers who reported complications during pregnancy. On the other hand, one fetal death was attributed to a miscarriage in a healthy mother with no reported pregnancy-related complication [36]. Moreover, it was noted that preeclampsia and DIC were the most prevalent complications. Narang et al. explained that infection with SARS-CoV-2 throughout pregnancy holds the possibility of initiating microvascular dysfunction by causing endotheliitis [65]. Furthermore, ischemia and vasoconstriction may ensue as a result of the microcirculatory dysfunction and systemic inflammation, which can further contribute to a pro-coagulopathic state [65]. Given that complications in pregnant women act as detrimental prognostic factors, it is crucial to pay close attention to the development of any laboratory abnormalities throughout pregnancy.
Due to the novelty of the subject, follow-up of COVID-19 positive first and second trimester mothers and their newborns is insufficient. The majority of 1st and 2nd trimester women who got pregnant near the start of the pandemic are yet to give birth or complete pregnancy. Such limitations restrict the assessment of complication development, outcomes (both maternal and neonatal), and course of pregnancies. The presented review provides a comprehensive take on patterns concerning COVID-19 contracted in 1st and 2nd trimesters, and allows for the construction of appropriate research on the topic.
In conclusion, considering that the majority of mothers who contracted COVID-19 during the 1st and 2nd trimesters are yet to complete their pregnancy, an evidently scarce amount of data is available concerning neonatal and maternal outcomes. Mothers were largely discharged from the hospital without any serious complications; however, placental inflammatory changes and neonatal death have been reported in a number of cases. Further research is required to determine whether or not contracting SARS-CoV-2 early on in pregnancy poses a consequential risk to either the mother or neonate.