Introduction
The World Health Organization has reported an astonishing 212,357,898 confirmed instances of coronavirus disease 2019 (COVID-19) globally. Among the nations profoundly impacted by this pandemic, Brazil distinguishes itself by holding the unfortunate third position in terms of case count and the second position in terms of fatalities, with a somber tally of 574,527 deaths [1]. As of August 2021, the Ministry of Health has meticulously documented the hospitalization of 2,558,360 patients diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2].
A stark and unsettling warning was disseminated through a COVID-19 bulletin originating from Brazil, foreshadowing an impending collapse of healthcare systems under the sheer weight of overwhelming demand. This dire scenario manifested as bed occupancy rates surged well beyond their designed capacity in all states, exceeding an alarming 100% [3]. The availability of both intensive care unit (ICU) beds and mechanical ventilators surfaced as a pivotal challenge confronting healthcare systems on a global scale. Significantly, the glaring disparity between the supply and demand of mechanical ventilators, precipitated by the unrelenting onslaught of SARS-CoV-2, gave rise to a pressing crisis. The scarcity of ventilatory support resources further compounded the strain experienced by medical facilities across the world [4].
For approximately 30 years, the helmet has served as an alternative to conventional interfaces. Nevertheless, it was exclusively during the pandemic period that this apparatus underwent adaptation for pressurization. This adaptation was achieved through the utilization of mechanical ventilation (MV) or direct connection to medical gas pipeline systems, employing low-flow meters that combine medical air and oxygen. This arrangement is complemented by the integration of a positive end-expiratory pressure (PEEP) valve.
The utilization of the pressurized helmet, in conjunction with the flow meter (FM) and PEEP valve (PV), presents an innovative approach akin to continuous positive airway pressure (CPAP). This methodology assumes a remarkable level of importance as a financially efficient resource, particularly when confronted with the prevailing scarcity of mechanical ventilators amidst the pandemic context [5–14].
Helmet CPAP gained widespread usage in Italy during the COVID-19 pandemic and found extensive application in numerous healthcare settings across Brazil. However, a notable gap exists in the literature regarding the established efficacy of employing flow meter-PEEP valve pressurization (FM-PV) for managing SARS-CoV-2 in adult patients [15–21], particularly within the context of the Brazilian population.
Hence, the primary objective of this study was to discern potential distinctions between employing non-invasive mechanical ventilation (NIV) with a helmet interface pressurized through FM-PV and utilizing pressurized MV. Our anticipation is that the outcomes from our research will furnish validation for a low-cost instrument, thereby alleviating the prevailing shortage of mechanical ventilators. This validation, in turn, will facilitate informed clinical decision-making and enhance overall precision in patient care.
Material and methods
Study design
This single-center randomized clinical trial was executed from June 2021 to September 2021 at the Hospital Professora Lydia Storópoli, a specialized institution in COVID-19 treatment situated in São Paulo City, within the premises of Universidade Nove de Julho – Campus Vergueiro. The trial’s registration was completed at ensaiosclinicos.gov.br (Registration No. RBR-8gfdkg4), and its execution adhered rigorously to the protocol endorsed by the Institutional Ethical Committee of Universidade Nove de Julho – Uninove (Approval No. 46677121.9.0000.5511). Furthermore, the trial was conducted in strict accordance with the principles outlined in the 1964 Helsinki Declaration, in addition to comparable ethical standards and instances. It is imperative to emphasize that every methodological aspect was meticulously carried out in compliance with the pertinent regulations and guidelines governing such endeavors.
Patients
All consecutive adult patients admitted to the ICU owing to acute hypoxemic respiratory failure underwent a comprehensive screening process for potential enrollment in the study. Originally designed to encompass patients diagnosed with COVID-19, the study’s scope was directed towards this specific population.
In this study, we enrolled individuals aged 18 years and older, with SARS-CoV-2 infection confirmed through reverse transcriptase-polymerase chain reaction (RT-PCR). Our inclusion criteria encompassed patients exhibiting the following attributes: adequate level of consciousness, utilization of oxygen therapy, a fraction of inspired oxygen (FiO2) ranging from 45% to 81% with escalating oxygen flow, a partial pressure of oxygen (PaO2) surpassing 60 mm Hg, a partial pressure of carbon dioxide equal to or less than 50 mm Hg, peripheral oxygen saturation (SpO2) below 93% with oxygen supply exceeding or equal to 6 l/min, and hemodynamic stability.
Conversely, patients who had an absolute contraindication to the utilization of non-invasive ventilation (NIV), those with a previous diagnosis of chronic obstructive pulmonary disease (COPD) featuring chronic carbon dioxide (CO2) retention or clinical manifestations of the ailment [22–24], individuals meeting palliative therapy criteria, those with hypoxemia unrelated to viral pneumonia, and those displaying severe indications of hypoxemia such as central cyanosis, diminished consciousness level, or pronounced psychomotor agitation, coupled with a respiratory rate (RR) exceeding 35 and an SpO2 below 80%, were excluded from the study.
Comprehensive information regarding all procedures and potential risks, including their exponential implications, was meticulously communicated to all patients (or their legal representatives) prior to the commencement of the study. Moreover, an exhaustive elucidation of the current research objectives was provided to ensure transparency. Subsequently, all participants or their respective representatives provided informed consent by signing the consent form, under the guidance of the ICU’s dedicated physical therapist at the inception of the study.
Recruitment
The sampling procedure was carried out consecutively, in accordance with the order of service. An initial assessment was conducted by a licensed ICU physician responsible for scrutinizing patients against the study’s eligibility criteria, following a standardized protocol designed for this purpose. Following these assessments, patients were referred to the physical therapy team to proceed with the study protocol. To gather patients’ clinical information and characteristics, a socio-demographic datasheet was employed for comprehensive data collection.
Randomization
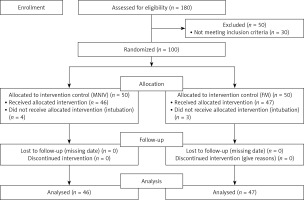
The recruitment of participants is depicted in the Consort diagram (Figure 1). The participants were allocated randomly to two groups at a 1 : 1 ratio: the ventilator + helmet group (VM), utilizing the 7Lives helmet from São Paulo, Brazil, and the flow meter + helmet group (FM-PV), also employing the 7Lives helmet from São Paulo, Brazil. Randomization was performed based on the place of admission; patients admitted to ICU-A were assigned to the MV group, while patients admitted to ICU-B were assigned to the FM-PV group.
Procedures
Each patient received central venous access for the purpose of administering sedation and analgesia, which consisted of morphine and dexmedetomidine. This regimen was designed to ensure the patient’s comfort during the duration of device usage, while maintaining the Richmond Agitation Sedation Scale (RASS) scores within the range of 0 to –2 [25]. To mitigate the risk of severe aerophagia-induced intestinal emesis and meteorism, prokinetic agents, specifically metoclopramide and simethicone, were administered.
Corticosteroid therapy was introduced for patients with a PaO2/FiO2 ratio between 200 and 150, involving the administration of dexamethasone. For patients with a PaO2/FiO2 ratio below 150 or those who did not respond adequately to initial dexamethasone treatment, the therapeutic approach encompassed the administration of methylprednisolone. It is imperative to note that all medication administration adhered strictly to medical prescriptions and protocols established by the attending physician team.
A nasoenteral tube was utilized to ensure adequate basal nutritional intake and to minimize masticatory effort. Additionally, an indwelling catheter was employed for various purposes, including reducing intra-abdominal pressure, optimizing diaphragm mechanics by maintaining an empty bladder, preventing unnecessary voiding efforts, managing fluid balance, and minimizing patient movement.
Following the preliminary assessments and screenings conducted by the medical team, patients who provided consent to participate were approached by the physiotherapy team for the purpose of device installation.
The control group (MV) underwent NIV using a mechanical ventilator, configured in a mode that enabled the manipulation of parameters such as FiO2, inspiratory pressure, PEEP, and indirectly, inspiratory flow. This was facilitated using equipment models such as the Mindray E3 and Beijing Aeonmed VG70.
In contrast, the intervention group (FM-PV) underwent NIV through a direct connection to the medical oxygen gas pipeline systems. This connection was established utilizing a 15 l flow meter (FM) and a positive end-expiratory pressure valve (PV), both of which were situated at the outlet port of the helmet. This setup allowed for direct adjustments of PEEP and indirect adjustments of FiO2 and inspiratory flow.
In both groups, four key parameters were subject to adjustment: (a) the inspiratory flow rate necessary to achieve adequate pressurization of the 7LivesR helmet, (b) the FiO2, (c) inspiratory pressure, and (d) PEEP. These adjustments were meticulously made while ensuring that each parameter maintained a minimum value sufficient to attain a peripheral oxygen saturation (SpO2) level equal to or exceeding 92%.
Intervention
Arterial blood gases
Blood gas samples were collected by the nursing team prior to interface installation, 2 h after helmet setup, and as part of the daily patient assessments until interface discontinuation. This systematic approach served the dual purpose of aiding SARS-CoV-2 diagnosis and informing necessary ventilatory adjustments based on blood gas findings, thereby contributing to therapeutic management (Figures 2).
Modified Borg scale and visual analog scale
The evaluation of the degree of respiratory distress was conducted through the utilization of the Modified Borg numerical scale [26]. Additionally, a visual analog scale, ranging from 0 to 10, was employed to gauge the patient’s comfort and level of claustrophobia. It is noteworthy that higher values on this scale correspond to increased severity of respiratory distress [27].
Respiratory and hemodynamic monitoring
A Dixtal monitor was employed to monitor vital parameters, encompassing heart rate (HR), peripheral oxygen saturation (SpO2), respiratory rate (RR), and blood pressure (BP).
The primary outcome was determined as the proportion of patients requiring endotracheal intubation within 28 days following enrollment in the study.
Secondary outcomes included various aspects: the number of days without respiratory support within the initial 28 days from enrollment, the number of days without invasive mechanical ventilation by day 28, in-ICU mortality, in-hospital mortality, daily mortality over a 28-day period, the duration of ICU stay, and the duration of hospital stay.
Statistical analysis
Based on an estimated effect size of d = 0.80, α level = 0.05, β level = 0.80, power = 0.80, we estimated that a total of 50 participants per group would be required. Sample calculation would provide ventilatory-support free days on a 28-day basis in the helmet group [22]. Therefore, we planned a 10% dropout rate of 110 patients, diagnosed with SARS-CoV-2.
The Shapiro-Wilk test was used to determine the normality of anthropometric, demographic, and clinical data distribution. Parametric data were expressed as mean ± standard deviation. Non-parametric data were expressed as median and interquartile interval. Categorical data are expressed as numbers (percentages).
For intragroup analysis (before and after the protocol), the paired T-test or Wilcoxon test was performed for parametric and non-parametric variables, respectively. For intergroup analysis, the T-test was used for independent data or the Mann-Whitney U test for parametric and non-parametric variables, and the χ2 test for categorical variables was used. Results were considered statistically significant when the p-value was ≤ 0.05. The number of days until intubation at each time point was analyzed using the Kaplan-Meier method with a 95% confidence interval. The log-rank test was used to compare curves. All data were tabulated and analyzed using IBM SPSS Statistics 22.0.
Results
Between June and September 2021, a total of 180 patients diagnosed with acute respiratory failure were admitted to two distinct ICUs, designated as ICU-A and ICU-B. Out of this initial pool, 100 patients were selected to participate in the study. Initially, 50 patients were assigned to the MV group, while an equivalent number were allocated to the FM-PV group. However, following the exclusion of 7 participants due to significant protocol deviations or data loss, the final analysis encompassed 46 patients in the MV group and 47 patients in the FM-PV group.
Patients’ demographic data are presented in Table I.
Table I
Demographic data and primary and secondary outcomes according to study group
All individuals enrolled in this study were confirmed to have a molecular diagnosis of COVID-19. However, due to the nature of the arterial blood gas sampling, which took place 2 h prior to and after the initiation of the protocol, determining the PaO2/FiO2 ratio before the protocol’s commencement for all patients was not feasible. This constraint emerged because patients were already receiving oxygen therapy through a non-rebreather mask, which precluded the accurate determination of the FiO2.
Nonetheless, the regular collection of daily arterial blood gas samples holds paramount importance. This practice ensures the maintenance of safe O2 and CO2 levels throughout the duration of helmet usage.
Primary outcome
There were no statistically significant differences between groups at the day 28 after randomization (p = 0.37). The intubation rate for the MV vs. FM-PV were 54.3 and 46.8%, respectively.
Secondary outcomes
This trial included 6 secondary outcomes: death rate and the average number of days in the following outcomes: helmet wearing, hospitalization, ICU stay, and device use until hospital discharge (Table I). The average number of days of helmet use was lower in the FM-PV group: 3.4 ±1.6 vs. 4.0 ±1.9. In addition, the mean number of hospitalization days was lower in the FM-PV group: 17.1 ±9.5 vs. 15.9 ±7.9. Similarly to the results described above, the MV group presented a higher mean number of days of hospitalization in the ICU than the FM-PV group: 8.3 vs. 12.4. The largest difference between groups was the percentage of intubation, which was approximately 10% higher in the MV group (54.3% vs. 46.8%). Finally, the mortality rate was higher in the MV group (47.8% vs. 45.8%).
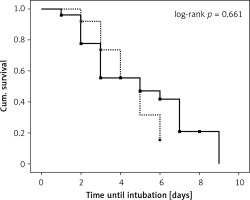
The proportion of patients who were not intubated was 43.9% (32/57) and 53.8% (30/65) in the MV and FM-PV groups, respectively. The survival was similar between groups (p = 0.661) with mean times until intubation of 7.9 (4.1–11.7 days) and 7.0 (5.2–8.8 days) for MV and FM-PV groups, respectively (presented in Figure 3).
Discussion
This is the first single-center randomized clinical trial aiming to investigate the utilization of non-invasive mechanical ventilation (NIMV) with a helmet interface pressurized using either a mechanical ventilator or an FM. The primary finding of this study did not reveal any statistically significant differences between the two groups.
However, it is important to highlight that the absence of disparities between the groups is a noteworthy outcome. This contrasts with other studies that have compared the effectiveness of helmets against different interfaces. In our study, the comparison focused on a different aspect – specifically, the same helmet interface pressurized using different devices, namely the mechanical ventilator and FM [22, 28]. Therefore, the primary aim of this investigation was to assess the efficacy of the devices employed for pressurizing the helmet interface.
The potential for integrating the helmet interface through direct access to medical gas pipeline systems has been recognized in a prior study. However, prior to the current literature review, this represents the first randomized clinical trial aimed at contrasting the effectiveness of pressurizing the helmet interface using an FM directly integrated into the medical gas pipeline systems [5, 29].
The global SARS-CoV-2 pandemic has placed an immense strain on healthcare systems worldwide, compelling nations to devise effective healthcare strategies and disseminate guidelines to govern the application of oxygen therapy and ventilatory support among COVID-19 patients. It is worth noting that the Italian Society of Pulmonology advocates the priority use of a helmet as the primary modality for NIV [30]. In contrast, in Brazil, the Ministry of Health recommends initiating NIV when oxygen therapy exceeds 6 l/min [31].
For instance, in the United Kingdom, a study including 206 COVID-19 patients revealed notable benefits linked to the prompt application of CPAP. Commencing within 4 days of hospital admission, this approach yielded a survival probability over 73% [32]. In stark contrast, the mortality rate among COVID-19 patients subjected to invasive mechanical ventilation in Brazil reaches 80% [33]. This glaring disparity accentuates the urgent requirement for carefully delineated protocols for non-invasive ventilatory support, underpinned by substantial scientific substantiation.
A study conducted within the Brazilian population revealed that 49% of hospitalized patients diagnosed with COVID-19 required NIV. Despite the absence of substantial evidence and advantages of NIMV in hypercapnic acute respiratory failure (hARF), a comprehensive protocol addressing the implementation and gradual cessation of this ventilatory support is notably lacking in the “Brazilian Guidelines” for managing COVID-19 patients undergoing hospital treatment. Furthermore, no reference is made to the sustained utilization of NIV with the helmet interface [29, 33].
Helmets represent a crucial and evidence-supported alternative [5, 22, 28, 29]. In comparison to other interfaces such as oronasal masks and high flow nasal cannulas, non-invasive ventilation (NIV) with helmets has exhibited a notably reduced rate of endotracheal intubation. This mode of ventilation has led to an increase in the number of days without mechanical ventilation, a decrease in ICU stays, and a decline in 90-day mortality [22, 28]. In their study, Patel et al. conducted a comparison between the intubation rates of positive pressure therapy using helmets versus face masks, revealing an intubation rate of 61.5% for face masks and 18.5% for helmets [28].
Recent research has highlighted that the notable reduction in the intubation rate can be partially elucidated by the effective delivery of higher levels of PEEP. This suggests that a more secure seal of the helmet around the neck enables the administration of increased airway pressures, attributed to the minimal presence of air leakage [28, 34]. Drawing from the pathophysiology of acute lung injury, we have formulated a hypothesis that optimal outcomes when utilizing the helmet interface may be linked to the potential for sustained positive pressure application over extended periods. It is well established that in the realm of ideal values, positive pressure therapy serves to reverse alveolar collapse, enhance ventilation, facilitate gas diffusion, mitigate the cyclical opening and closing of alveoli, and reduce the incidence of patient self-induced lung injury (P-SILI), thereby improving oxygenation and diminishing respiratory effort. Consequently, this cascade of effects contributes to a decreased intubation rate [28, 34, 35].
Despite the fact that the helmet interface has been in use for three decades, its application in the context of non-invasive ventilation management (NIVM) is a relatively recent development. Consequently, it necessitates careful attention to its implementation as well as a comprehensive analysis of its inherent limitations. For instance, the helmet’s notable internal volume can potentially lead to CO2 rebreathing; however, this concern can be alleviated by utilizing higher gas flow rates [17, 28, 36]. Additionally, it is important to note that arterial blood collection becomes a daily requirement when utilizing the helmet interface [28]. The matter of patient-ventilator asynchrony also emerges as a key consideration during helmet usage [28]. However, it is crucial to recognize that asynchrony is not unique to this particular interface; elevated rates of asynchrony are frequently observed across various interfaces during the application of NIMV [37]. Addressing this lack of synchronization between the patient’s respiratory efforts and the delivered ventilatory support stands as a critical determinant of the overall effectiveness of the therapy [38].
In terms of cost considerations, an audit conducted by the Comptroller General (CGU) during the pandemic revealed that the average expense borne by states and municipalities for 75% of the purchased mechanical ventilators reached up to US$ 22,500 per unit [39]. In stark contrast, the expenditure associated with each FM for the host hospital in the present study was approximately US$ 40.
Nonetheless, this trial exhibits a multitude of strengths. Firstly, the study presents a solution that is economically efficient. Secondly, it introduces a non-invasive therapeutic alternative that could hold significant economic implications for healthcare provisioning worldwide, especially in less developed nations.
In conclusion, this is the first study to show that pressurizing the helmet interface using FM and PV is as effective as MV, providing a low-cost therapeutic alternative.