Introduction
Nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) are metabolic disorders that share common pathogenic mechanisms. The prevalence rates of NAFLD and T2DM are 25.24% and 22.51%, respectively [1]. However, T2DM increases the prevalence of NAFLD to as high as 70% [2]. Conversely, the presence of NAFLD is a predictive factor for the development of T2DM. The study of Eun et al. [3] showed that the prevalence of severe NAFLD is higher in patients with T2DM than in those with normal glucose tolerance. This association could be attributed to the pathogenic mechanisms of defective lipid metabolism and hepatic triglyceride accumulation as well as the progression of insulin resistance with compensatory hyperinsulinemia to β-cell dysfunction [4]. Worse, NAFLD and T2DM increase the prevalence and severity of the complications [5]. In addition, these diseases lead to vascular complications and aggravate NAFLD to liver cirrhosis and hepatocellular carcinoma [6, 7]. The treatment of NAFLD and T2DM has remained a clinical challenge for several years [8].
Dipeptidyl peptidase-4 (DPP-4) inhibitor (DPP4i) and sulfonylurea (SU) are the two types of second-phase glucose-lowering drugs used to treat patients with T2DM after metformin treatment. Apart from sitagliptin, vildagliptin, and alogliptin [9–11], another type of DPP-4i, linagliptin, is advantageous in reducing the risk of hypoglycemia and in achieving weight neutrality [12]. In animal experiments on NAFLD with diabetes, linagliptin has been shown to exert anti-inflammatory and antisteatotic effects [13, 14]. Glimepiride, a long-acting antidiabetic drug that belongs to third-generation SU, is used to treat T2DM [15]. The drug binds to the SU receptors on the surface of pancreatic β-cells, which prompts the closure of KATP channels and the influx of Ca2+, thereby promoting the release of insulin and inhibiting hepatic glucose synthesis. In addition to the effect of lowering blood glucose, glimepiride offers several potential benefits over currently available SUs, including antioxidative, anti-inflammatory, angiogenic, and endocrine effects [16]. Studies on efficacy comparisons between linagliptin and glimepiride have been widely conducted in recent years.
MicroRNAs (miRNAs) are a kind of small noncoding RNA molecules (21–25 nucleotides in length) that regulate gene expression at the post-transcriptional level and participate in various critical biological processes [17–19]. miRNA regulates human genes that are involved in almost all cellular processes [20, 21] and is linked to the diagnosis or prognosis of numerous diseases, including T2DM [22–24]. Studies have revealed that miR-210 is related to hepatic function and dysregulation in T2DM [25, 26]. However, whether miR-210 serves as a diagnostic and prognostic biomarker in patients with T2DM and NAFLD treated using glimepiride and linagliptin is yet to be elucidated.
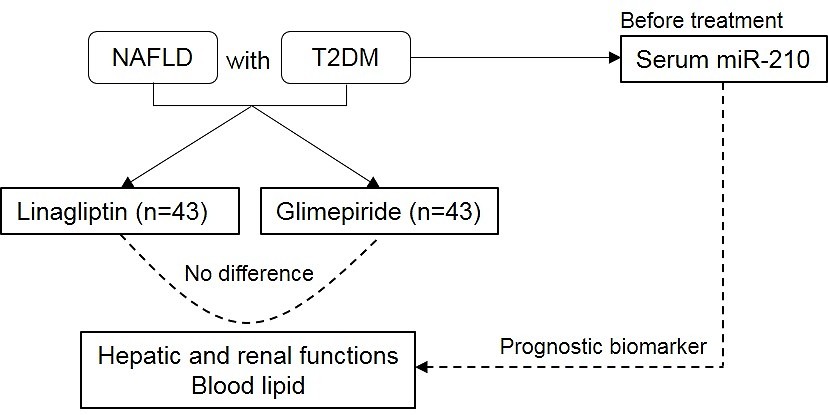
A random, controlled study was conducted in which the effect of linagliptin was compared with that of glimepiride in lowering blood glucose, improving fatty hepatic disease, and influencing lipid metabolism markers in patients with NAFLD and T2DM. Moreover, the role of miR-210 as a diagnostic or prognostic biomarker in patients with NAFLD and T2DM treated with glimepiride and linagliptin was assessed. This study strives to document the linagliptin-related clinical experience and data in Chinese patients and provide clinical evidence for the drug’s hypoglycemic and islet function-protecting effects.
Material and methods
Patients
A total of 86 NAFLD with T2DM patients (54 men, 32 women) who visited the University of Chinese Academy of Sciences Shenzhen Hospital from October 2019 to October 2020 were enrolled in this study. All patients provided signed informed consent forms. This study was approved by the ethics committee of the University of Chinese Academy of Sciences Shenzhen Hospital (accession number: LL-KT-201804).
Inclusion and exclusion criteria
The main inclusion criteria for the subjects were as follows: patients of age 40–70 years; body mass index (BMI) of 18.0–25.0 kg/m2; patients previously diagnosed as having T2DM with NAFLD in accordance with the Guidelines for the Management of the University of Chinese Academy of Sciences Shenzhen Hospital; computed tomography (CT) diagnosis showing diffused hepatic density and decreased diffuse liver density decrease, with a hepatic/renal CT ratio of ≤ 1.0. The histological changes of hepatic biopsy met the pathological diagnostic criteria of fatty hepatic diseases; the patient did not have a history of drinking or consumed alcoholic beverages within the limit of < 70 g/week; suitable for the use of linagliptin and glimepiride.
The main exclusion criteria for the subjects were as follows: patients who took anti-diabetic drugs other than metformin within the last 3 months; patients with uncontrolled hypertension; patients affected by myocardial infarction or stroke within the past 6 months; impaired hepatic or renal function; patients with severe hypoglycemia within the past 6 months.
Grouping and treatments
The patients were randomly assigned to two groups and treated with either linagliptin (5 mg/daily) or glimepiride (2 mg/daily) for 6 months. All patients were provided the same dietary guidance.
Observation indicators
The clinical baseline data of gender, age, weight, height, BMI, visceral fat (cm2), subcutaneous fat (cm2), and diagnosis time (month) were recorded. The laboratory parameters were measured, which included the levels of fasting blood glucose (mmol/l), 2-h post-breakfast blood glucose (2h-PBG; mmol/l), fasting insulin (µIU/ml), 2-h post-breakfast insulin (µIU/ml), fasting C-peptide (µg/l), 2-h post-breakfast C-peptide (µg/l), glycated hemoglobin (HbA1c; %), alanine aminotransferase (ALT; IU/l), aspartate aminotransferase (AST; IU/l), alkaline phosphatase (ALP; IU/l), γ-glutamyl transpeptidase (GGT; IU/l), albumin (ALB; g/l), blood urea nitrogen (BUN; mmol/l), creatinine (CR; µmol/l), uric acid (UA; µmol/l), total cholesterol (TG; mmol/l), triglycerides (TC; mmol/l), high-density lipoprotein cholesterol (HDL; mmol/l), and low-density lipoprotein cholesterol (LDL; mmol/l), along with the CT value of hepatic and renal functions. All laboratory parameters were tested by using an autoanalyzer (AU5800, Beckman Coulter, Miami, FL, USA). In addition, the areas of visceral and subcutaneous adipose tissues were measured and calculated using the HDS-2000 DUALSCAN fat-measuring device (OMRON, Kyoto, Japan) according to its operating instructions.
Real-time quantitative PCR (qPCR)
The serum in the patients was isolated from total blood (2 ml) before and after treatments. The total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The primers of miR-210 and U6 were designed and synthesized by Sangon (Shanghai, China). Then, the qRT-PCR analysis was conducted by applying the miRNA Universal SYBR qPCR Master Mix (Cat#:MQ101) provided by Vazyme Biotech Co. Ltd. (Nanjing, China). Finally, the expression of miR-210 was tested on the ABI 7900 System (Foster City, CA, USA) and calculated using the 2–ΔΔCt method [27] compared with U6.
Statistical analysis
All experiments were conducted in triplicate. IBM SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, IBM, Armonk, NY, USA) was used for statistical analyses. According to the expression of miR-210, the samples were assigned to two groups using the medium expression. The Shapiro-Wilk test was performed to examine the normality of the data. Changes in the basic clinical data and laboratory parameters between the groups were tested by paired t-test. Data were expressed as the mean ± standard deviation (SD). P < 0.05 was considered to indicate a statistically significant difference. Pearson’s correlation test was performed to obtain the correlation between the expression of miR-210 and the experimental data.
Results
Baseline characteristics
A total of 86 patients were randomly assigned to two groups and were treated with either glimepiride or linagliptin. Only diagnosis time was significantly different between the two groups (p = 0.044; Table I). No significant differences were noted in the laboratory parameters of patients treated using linagliptin compared with those treated using glimepiride, which indicated that the baseline clinical characteristics were balanced.
Table I
Baseline clinical characteristics of NAFLD with T2DM patients
| Items | Glimepiride group | Linagliptin group | P-value |
|---|---|---|---|
| Gender (male/female) | 33/10 | 21/22 | – |
| Age [years] | 55.77 ±7.83 | 55.81 ±8.43 | 0.979 |
| Height [cm] | 163.36 ±8.44 | 160.77 ±7.90 | 0.145 |
| Weight [kg] | 66.14 ±9.74 | 62.61 ±10.15 | 0.104 |
| BMI [kg/m2] | 24.73 ±2.34 | 24.15 ±2.53 | 0.270 |
| Visceral fat [cm2] | 93.17 ±23.84 | 85.67 ±33.67 | 0.236 |
| Subcutaneous fat [cm2] | 203.77 ±158.01 | 171.40 ±51.46 | 0.205 |
| Diagnosis time [months] | 99.26 ±68.96 | 70.47 ±61.27 | 0.044* |
| Fasting blood glucose [mmol/l] | 7.84 ±2.57 | 7.57 ±1.76 | 0.562 |
| 2 h-PBG [mmol/l] | 12.34 ±5.07 | 11.73 ±4.46 | 0.553 |
| Fasting insulin [µIU/ml] | 10.55 ±7.15 | 11.70 ±9.31 | 0.521 |
| 2 h PB insulin [µIU/ml] | 47.33 ±33.33 | 46.46 ±38.42 | 0.911 |
| Fasting C-peptide [µg/l] | 2.58 ±2.43 | 2.19 ±0.99 | 0.325 |
| 2 h PB C-peptide [µg/l] | 6.59 ±4.10 | 5.56 ±2.65 | 0.173 |
| HbA1c (%) | 7.61 ±2.06 | 7.15 ±1.31 | 0.221 |
| ALT [IU/l] | 47.6 ±12.36 | 49.93 ±12.32 | 0.385 |
| AST [IU/l] | 41.88 ±13.49 | 39.98 ±11.83 | 0.488 |
| ALT/AST | 1.37 ±1.18 | 1.37 ±0.59 | 0.982 |
| ALP [IU/l] | 81.00 ±19.49 | 79.74 ±25.01 | 0.796 |
| GGT [IU/l] | 27.58 ±15.44 | 29.35 ±24.13 | 0.687 |
| ALB [g/l] | 46.17 ±5.38 | 45.67 ±3.08 | 0.600 |
| BUN [mmol/l] | 5.75 ±3.22 | 5.61 ±1.67 | 0.795 |
| CR [µmol/l] | 85.91 ±124.42 | 67.98 ±20.34 | 0.354 |
| UA [µmol/l] | 346.85 ±89.99 | 346.3 ±82.84 | 0.977 |
| TG [mmol/l] | 3.37 ±4.10 | 2.97 ±4.38 | 0.660 |
| TC [mmol/l] | 5.18 ±1.34 | 5.02 ±1.42 | 0.597 |
| HDL [mmol/l] | 1.17 ±0.41 | 1.17 ±0.26 | 0.985 |
| LDL [mmol/l] | 2.82 ±0.87 | 2.69 ±0.66 | 0.430 |
| CT value of hepatic | 41.66 ±7.26 | 42.59 ±9.67 | 0.614 |
| CT value of renal | 51.13 ±6.17 | 52.61 ±8.94 | 0.373 |
| Hepatic/renal CT ratio | 0.82 ±0.11 | 0.81 ±0.12 | 0.855 |
T2DM – type 2 diabetes mellitus, NAFLD – non-alcoholic fatty liver disease, BMI – body mass index, 2 h-PBG – 2 h post-breakfast (PB) glucose, HbA1c – glycated hemoglobin, ALT – alanine aminotransferase, AST – aspartate aminotransferase, ALP – alkaline phosphatase, GGT – γ-glutamyl transpeptidase, ALB – albumin, BUN – blood urea nitrogen, CR – creatinine, UA – uric acid, TG – total cholesterol, TC – triglycerides, HDL – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol, CT – computed tomography. Values are expressed as mean ± SD.
Baseline characteristics based on the miR-210 expression level
The patients were classified in two groups based on the expression level of miR-210. After grouping, the baseline characteristics and laboratory parameters showed significant differences in terms of age, weight, BMI, fasting blood glucose level, and hepatic/renal CT ratio (p < 0.05; Table II). Furthermore, the expression level of miR-210 was negatively correlated with age (weak correlation, r = –0.361, p = 0.001) and positively correlated with weight (weak correlation, r = 0.403, p < 0.001) and BMI (moderate correlation, r = 0.504, p < 0.001). Compared with the low-expression group, the age of patients in the high-expression group was significantly lower (p = 0.001; Table II). Moreover, the weight of patients in the low-expression group was significantly lower than that in the high-expression group (p < 0.001), which resulted in a corresponding lower BMI in patients in the low-expression group (p < 0.001).
Table II
Baseline clinical characteristics based on the medium expression level of miR-210
| Items | Low | High | P-value | Pearson r correlation |
|---|---|---|---|---|
| Gender (male/female) | 24/19 | 30/13 | – | –0.144 |
| Age [years] | 58.70 ±7.24 | 52.88 ±7.93 | 0.001 | –0.361** |
| Height [cm] | 160.91 ±8.89 | 163.22 ±7.44 | 0.194 | 0.141 |
| Weight [kg] | 60.36 ±9.78 | 68.40 ±8.68 | < 0.001 | 0.403*** |
| BMI [kg/m2] | 23.22 ±2.04 | 25.67 ±2.21 | < 0.001 | 0.504*** |
| Visceral fat [cm2] | 90.96 ±31.70 | 87.88 ±26.85 | 0.629 | –0.053 |
| Subcutaneous fat [cm2] | 193.27 ±157.14 | 181.90 ±58.24 | 0.657 | –0.049 |
| Diagnosis time [month] | 79.81 ±63.12 | 89.91 ±69.99 | 0.484 | 0.076 |
| Fasting blood glucose [mmol/l] | 7.17 ±1.58 | 8.23 ±2.58 | 0.024 | 0.243** |
| 2 h-PBG [mmol/l] | 11.20 ±4.10 | 12.86 ±5.25 | 0.106 | 0.176 |
| Fasting insulin [µIU/ml] | 11.34 ±9.28 | 10.90 ±7.23 | 0.807 | -0.027 |
| 2 h PB insulin [µIU/ml] | 46.77 ±39.70 | 47.02 ±31.8 | 0.974 | 0.004 |
| Fasting C-peptide [µg/l] | 2.19 ±1.10 | 2.59 ±2.38 | 0.322 | 0.108 |
| 2 h PB C-peptide [µg/l] | 5.63 ±2.91 | 6.52 ±3.94 | 0.238 | 0.129 |
| HbA1c (%) | 7.07 ±1.22 | 7.69 ±2.09 | 0.094 | 0.182 |
| ALT [IU/l] | 46.79 ±11.85 | 50.74 ±12.61 | 0.138 | 0.161 |
| AST [IU/l] | 39.26 ±12.06 | 42.60 ±13.14 | 0.222 | 0.133 |
| ALT/AST | 1.30 ±0.56 | 1.43 ±1.19 | 0.522 | 0.070 |
| ALP [IU/l] | 79.49 ±22.46 | 81.26 ±22.36 | 0.716 | 0.04 |
| GGT [IU/l] | 28.77 ±23.87 | 28.16 ±15.88 | 0.890 | -0.015 |
| ALB [g/l] | 45.90 ±2.34 | 45.95 ±5.75 | 0.957 | 0.006 |
| BUN [mmol/l] | 5.61 ±1.45 | 5.75 ±3.33 | 0.802 | 0.027 |
| CR [µmol/l] | 67.09 ±15.62 | 86.79 ±124.97 | 0.308 | 0.111 |
| UA [µmol/l] | 360.28 ±82.63 | 332.87 ±88.02 | 0.140 | -0.16 |
| TG [mmol/l] | 2.65 ±4.33 | 3.69 ±4.08 | 0.253 | 0.125 |
| TC [mmol/l] | 4.92 ±1.38 | 5.28 ±1.36 | 0.220 | 0.134 |
| HDL [mmol/l] | 1.13 ±0.24 | 1.21 ±0.42 | 0.321 | 0.108 |
| LDL [mmol/l] | 2.71 ±0.71 | 2.81 ±0.83 | 0.558 | 0.064 |
| CT value of hepatic | 40.81 ±10.19 | 43.43 ±6.27 | 0.155 | 0.155 |
| CT value of renal | 52.91 ±9.15 | 50.83 ±5.75 | 0.211 | -0.136 |
| Hepatic/renal CT ratio | 0.77 ±0.13 | 0.86 ±0.09 | 0.001 | 0.361** |
T2DM – type 2 diabetes mellitus, NAFLD – non-alcoholic fatty liver disease, BMI – body mass index, 2 h-PBG – 2 h post-breakfast (PB) glucose, HbA1c – glycated hemoglobin, ALT – alanine aminotransferase, AST – aspartate aminotransferase, ALP – alkaline phosphatase, GGT – γ-glutamyl transpeptidase, ALB – albumin, BUN – blood urea nitrogen, CR – creatinine, UA – uric acid, TG – total cholesterol, TC – triglycerides, HDL – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol, CT – computed tomography. Values are expressed as mean ± SD.
The miR-210 expression level was positively correlated with fasting blood glucose level (weak correlation, r = 0.243, p = 0.024) and hepatic/renal CT ratio (weak correlation, r = 0.361, p = 0.001). No significant differences were noted in other parameters. The fasting blood glucose level and hepatic/renal CT ratio were significantly lower in the low-expression group compared with those in the high-expression group.
Effects of linagliptin on patients with NAFLD and T2DM compared with those of glimepiride
After the trial, the effects of linagliptin on NAFLD with T2DM were comparatively evaluated with those of glimepiride. Patients treated with linagliptin exhibited a lower fasting blood glucose level than those who received glimepiride treatment (6.87 ±1.56 vs. 6.25 ±1.12, mmol/l; p = 0.039; Table III). However, no significant differences were observed in terms of 2h-PBG, fasting insulin, 2 h post-breakfast insulin, fasting C-peptide, or 2 h post-breakfast C-peptide levels. The glimepiride and linagliptin treatments did not result in any significant differences in hepatic and renal functions or blood lipid parameters. These results indicated that the drugs were comparable in improving the hepatic and renal functions and blood lipid levels.
Table III
Effects of linagliptin compared with glimepiride in NAFLD patients with T2DM
| Items | Glimepiride group | Linagliptin group | P-value | Pearson r correlation |
|---|---|---|---|---|
| Fasting blood glucose [mmol/l] | 6.87 ±1.56 | 6.25 ±1.12 | 0.030 | –0.224* |
| 2 h-PBG [mmol/l] | 11.07 ±4.59 | 10.89 ±4.10 | 0.847 | –0.021 |
| Fasting insulin [µIU/ml] | 11.60 ±5.46 | 11.88 ±5.48 | 0.810 | 0.026 |
| 2 h PB insulin [µIU/ml] | 56.89 ±40.58 | 57.23 ±31.88 | 0.965 | 0.005 |
| Fasting C-peptide [µg/l] | 3.03 ±1.21 | 3.38 ±2.34 | 0.394 | 0.093 |
| 2 h PB C-peptide [µg/l] | 6.69 ±2.71 | 7.19 ±2.89 | 0.411 | 0.09 |
| HbA1c (%) | 7.08 ±1.18 | 7.41 ±1.61 | 0.275 | 0.119 |
| ALT [IU/l] | 33.51 ±13.68 | 31.96 ±13.08 | 0.594 | –0.058 |
| AST [IU/l] | 32.62 ±8.63 | 30.28 ±8.62 | 0.212 | –0.136 |
| ALT/AST | 1.06 ±0.43 | 1.13 ±0.50 | 0.536 | 0.068 |
| ALP [IU/l] | 71.36 ±21.48 | 74.88 ±21.02 | 0.445 | 0.084 |
| GGT [IU/l] | 27.66 ±23.52 | 27.63 ±15.81 | 0.994 | –0.001 |
| ALB [g/l] | 45.36 ±3.29 | 45.98 ±6.33 | 0.573 | 0.062 |
| BUN [mmol/l] | 5.36 ±1.53 | 5.00 ±1.69 | 0.304 | –0.112 |
| CR [µmol/l] | 64.94 ±16.20 | 84.39 ±125.22 | 0.315 | 0.11 |
| UA [µmol/l] | 343.41 ±83.52 | 317.99 ±92.17 | 0.184 | –0.145 |
| TG [mmol/l] | 2.08 ±1.98 | 1.95 ±1.45 | 0.716 | –0.04 |
| TC [mmol/l] | 4.66 ±1.08 | 4.70 ±1.19 | 0.870 | 0.018 |
| HDL [mmol/l] | 1.09 ±0.33 | 1.12 ±0.49 | 0.706 | 0.041 |
| LDL [mmol/l] | 2.58 ±0.75 | 2.75 ±0.90 | 0.328 | 0.107 |
2 h-PBG – 2 h post-breakfast (PB) glucose, HbA1c – glycated hemoglobin, ALT – alanine aminotransferase, AST – aspartate aminotransferase, ALP – alkaline phosphatase, GGT – γ-glutamyl transpeptidase, ALB – albumin, BUN – blood urea nitrogen, CR – creatinine, UA – uric acid, TG – total cholesterol, TC – triglycerides, HDL – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol. Values are expressed as mean ± SD.
Identification of miR-210 as a biomarker in patients with NAFLD and T2DM
The expression level of miR-210 varied significantly after the treatment. This level was positively correlated with the levels of fasting blood glucose (weak correlation, r = 0.272, p = 0.011) and 2h-PBG (weak correlation, r = 0.245, p = 0.023; Table IV). Compared with patients who exhibited low expression levels of miR-210, those who showed high expression levels had higher fasting blood glucose levels (6.93 ±1.54 vs. 6.19 ±1.11, mmol/l; p = 0.011). Moreover, the level of fasting insulin was negatively correlated with the expression level of miR-210 (weak correlation, r = –0.224, p = 0.038). Compared with the baseline characteristics (Table II), 2h-PBG and fasting insulin levels varied non-significantly in the low- and high-expression groups.
Table IV
Clinical characteristic comparison based on miR-210 expression after treatments with linagliptin and glimepiride
| Items | Low | High | P-value | Pearson r correlation |
|---|---|---|---|---|
| Fasting blood glucose [mmol/l] | 6.19 ±1.11 | 6.93 ±1.54 | 0.011 | 0.272* |
| 2 h-PBG [mmol/l] | 9.92 ±3.38 | 12.03 ±4.92 | 0.023 | 0.245* |
| Fasting insulin [µIU/ml] | 12.95 ±5.86 | 10.52 ±4.75 | 0.038 | –0.224* |
| 2 h post-breakfast insulin [µIU/ml] | 55.52 ±30.10 | 58.60 ±41.87 | 0.696 | 0.043 |
| Fasting C-peptide [µg/l] | 3.58 ±2.39 | 2.84 ±1.01 | 0.065 | –0.200 |
| 2 h post-breakfast C-peptide [µg/l] | 7.29 ±2.74 | 6.59 ±2.84 | 0.251 | –0.125 |
| HbA1c (%) | 7.37 ±1.52 | 7.13 ±1.30 | 0.431 | –0.086 |
| ALT [IU/l] | 26.93 ±9.79 | 38.54 ±13.95 | 0.000 | 0.438** |
| AST [IU/l] | 30.90 ±7.84 | 32.00 ±9.46 | 0.557 | 0.064 |
| ALT/AST | 0.92 ±0.35 | 1.27 ±0.50 | 0.000 | 0.382** |
| ALP [IU/l] | 72.42 ±21.68 | 73.82 ±20.94 | 0.762 | 0.033 |
| GGT [IU/l] | 30.68 ±25.68 | 24.61 ±11.16 | 0.159 | –0.153 |
| ALB [g/l] | 45.48 ±6.21 | 45.86 ±3.52 | 0.730 | 0.038 |
| BUN [mmol/l] | 5.06 ±1.58 | 5.30 ±1.65 | 0.495 | 0.075 |
| CR [µmol/l] | 85.73 ±124.62 | 63.60 ±18.85 | 0.253 | –0.125 |
| UA [µmol/l] | 334.18 ±99.69 | 327.22 ±76.41 | 0.717 | –0.040 |
| TG [mmol/l] | 1.58 ±1.12 | 2.45 ±2.09 | 0.018 | 0.255* |
| TC [mmol/l] | 4.59 ±1.10 | 4.77 ±1.16 | 0.479 | 0.077 |
| HDL [mmol/l] | 1.13 ±0.50 | 1.08 ±0.31 | 0.537 | –0.068 |
| LDL [mmol/l] | 2.65 ±0.89 | 2.67 ±0.77 | 0.903 | 0.013 |
2 h-PBG – 2 h post-breakfast blood glucose, HbA1c – glycated hemoglobin, ALT – alanine aminotransferase, AST – aspartate aminotransferase, ALP – alkaline phosphatase, GGT – γ-glutamyl transpeptidase, ALB – albumin, BUN – blood urea nitrogen, CR – creatinine, UA – uric acid, TG – total cholesterol, TC – triglycerides, HDL – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol. Values are expressed as mean ± SD.
Regarding hepatic function parameters, ALT and ALT/AST differed significantly (Table II). The levels of ALT (26.93 ±9.79 vs. 38.54 ±13.95, IU/l) and ALT/AST (0.92 ±0.35 vs. 1.27 ±0.50) were significantly lower in the low-expression group compared with those in the high-expression group (p < 0.001). The levels of ALT (weak correlation, r = 0.438) and ALT/AST (weak correlation, r = 0.382) were positively correlated with miR-210 expression.
However, no significant differences were observed in kidney function. The level of TG was positively correlated with the level of miR-210 expression (weak correlation, r = 0.255; p = 0.018). Collectively, these results signify that the miR-210 expression level might be associated with blood glucose and blood lipid levels and hepatic function.
Discussion
Patients with NAFLD tend to have components of metabolic syndrome, including T2DM, which leads to severe clinical outcomes, such as advanced hepatic fibrosis and mortality [28, 29]. In this study, the effect of linagliptin on NAFLD with T2DM was compared with that of glimepiride. Moreover, the role of miR-210 as a prognostic biomarker was evaluated. The following are the findings of the study: (1) When compared with glimepiride, linagliptin showed no difference in improving hepatic and renal functions and lowering the blood lipid level. (2) The miR-210 expression level might be a biomarker that indicates the prognosis of T2DM, hepatic function, and blood lipid level. The results confirmed the role of miR-210 as a prognostic biomarker when treated with linagliptin and glimepiride.
In this study, the effects of linagliptin on lowering the blood glucose and lipid levels, improving the fatty hepatic disease and weight, and promoting the hepatic or renal functions were investigated. As an SU drug, glimepiride exhibits various effects, such as antioxidative, anti-inflammatory, angiogenic, and endocrine effects [16]. The drug has also been shown to increase insulin sensitivity in patients with T2DM. Studies have suggested that the increase in adiponectinemia is associated with increased insulin sensitivity [30]. However, the decrease in insulinemia when treated with glimepiride is considered to be associated with the increased level of circulating adiponectin. Furthermore, a high level of adiponectin is inversely correlated with alterations in HbA1c, thereby lowering insulin requirement and improving glycemic control [31]. As a DPP-4i drug, linagliptin has also been widely used in patients with T2DM and NAFLD owing to its anti-inflammatory and antisteatotic activities [13, 14].
The effects and safety of linagliptin vs. glimepiride on T2DM have been widely studied previously. For example, Rosenstock et al. [32] found that linagliptin was beneficial in lowering the incidence of hypoglycemic events and was a noninferior risk factor for a composite cardiovascular outcome compared with glimepiride. A similar conclusion was also drawn in other studies [33–36]. In this study, the HbA1c level was similar in the two groups, which agreed with the studies mentioned above. However, linagliptin showed a better glucose-lowering effect than glimepiride as a result of better control of the fasting blood glucose level. The former studies were focused on adverse events and glucose control, and few studies have explored the hepatic and renal functions as well as blood lipid levels. This study concluded that linagliptin was not more beneficial than glimepiride, except in terms of lowering the blood glucose level.
The effect of miR-210 on T2DM has been extensively studied. For example, miR-210 has potential value to serve as a biomarker for T2DM with coronary artery disease [37]. The miR-210 levels are higher in patients with newly diagnosed T2DM than in those with normal glucose tolerance [38]. However, none of the studies have revealed the functions of miR-210 in NAFLD with T2DM. miR-210 has been established to be a hepatic-related miRNA that is positively correlated with weight, BMI, percentage of fat mass, and glucose level, especially with hepatic transaminases, which further supports the association between these serum miRNAs and hepatic dysfunction [39]. Considering the role of miR-210 in liver function, we wonder whether it is one of the biomarkers of NAFLD with T2DM that indicate hepatic function. The expression levels of miR-210 were found to be related to blood glucose and lipid levels and hepatic function, which indicates that it can be used as a prognostic biomarker for NAFLD with T2DM treated using linagliptin and glimepiride. Our previous study also showed that the circulating miR-210 level was elevated in T2DM and that a high level might be implicated in the occurrence and development of the disease and its complications [40]. Similarly, Pichu et al. observed that the miR-210 expression was increased both in the circulation and in tissue biopsies derived from patients with diabetic foot ulcer [41]. Furthermore, a study documented that the serum expression level of miR-210 was higher in patients with diabetic retinopathy than that in patients with diabetes mellitus without diabetic retinopathy and in healthy controls [42]. However, the study by Zhou et al. showed that miR-210 expression was downregulated in the erythrocytes, which induced endothelial dysfunction in T2DM [26]. A recent study suggested that the microenvironment of the adipose tissue significantly increased the expression of miR-210-3p in adipose tissue macrophages and subsequently induced adipose tissue inflammation and insulin resistance [43]. Based on our previous report [44] and the findings of this study, miR-210 appears to play a pertinent role in lipid metabolism and storage. Unfortunately, the sample size in this study was inadequate. Furthermore, the differences in the diagnosis time between the two groups is an important limitation of this study. In brief, the mechanism of the role of miR-210 in different cells needs further research.
In conclusion, based on the findings, linagliptin showed no difference in improving the hepatic and renal functions and in lowering the blood lipid level when compared with glimepiride. Importantly, the expression levels of miR-210 were related to blood glucose and lipid levels and hepatic function, thereby indicating that miR-210 could be used as a prognostic biomarker for NAFLD with T2DM treated using linagliptin and glimepiride. This study confirms the role of linagliptin compared with glimepiride and provides a novel prognostic biomarker for NAFLD with T2DM treated using linagliptin and glimepiride.