Introduction
Neonatal respiratory distress syndrome (NRDS) refers to acute and progressive anoxic respiratory failure in neonates caused by various external and internal pathogenic factors [1]. NRDS is one of the most important causes of death in neonatal intensive care units (NICU) [2, 3]. Abnormality of endogenous pulmonary surfactant (PS) is an important cause of NRDS. Lack of PS or abnormal function in NRDS results in an imbalance of ventilation/blood flow, decreased pulmonary compliance, and severe hypoxaemia [4, 5]. Studies have found that PS replacement therapy can significantly improve the morbidity and mortality of NRDS, and PS has become the main treatment of NRDS [6].
At present, there are three kinds of PS preparations for clinical application at home and abroad: 1) Containing natural PS protein SP-B and SP-C, including surfactant A, calfactant extracted from bovine lung lavage, poractant extracted from whole lung, and beractant extracted from whole bovine lung. 2) Containing synthetic protein, including synthetic SP-B peptide and DPPC phospholipid components, called lucinactant, also known as KL-4. 3) Colfosceril, which contains no protein and is widely used, consisting of phospholipids, hexadecanol, and tetrabutanol [7, 8].
Due to the lack of large sample randomised controlled studies; NRDS has not yet had an ideal NRDS treatment plan. Multiple randomised controlled trials (RCTs) were conducted to study exogenous PS in the treatment of NRDS, but its quality and efficacy were not systematically evaluated. We conducted a network meta-analysis to determine efficacy of PS in NRDS treatment, and to provide an evidenced-base reference for clinical use.
Material and methods
Search strategy
MEDLINE, Embase, The Cochrane Library, and Clinical Trials databases were electronically searched to collect RCT of antihypertensive drugs and hyperkalaemia events in patients with diabetic nephropathy from inception to January 2019. In addition, the reference to the published research was traced back to supplement the relevant literature. Two reviewers independently screened literature, extracted data, and assessed the risk bias of the included studies. The search was performed by means of a combination of subject words and free words, and appropriate adjustments were made according to different databases. The search terms included: Neonatal respiratory distress syndrome, NRDS, Infantile respiratory distress syndrome, Respiratory distress syndrome in infant, Pulmonary surfactant-associated protein A, Pulmonary surfactant-associated protein B, Pulmonary surfactant-associated protein C, Pulmonary surfactant-associated protein D, Pulmonary surfactant-associated proteins, Survanta, Alveofact, Infasurf, Curosurf, Surfaxin, Exosurf, Randomized controlled trials, RCT.
Inclusion and exclusion criteria
We only include randomised controlled trials, regardless of whether or not to refer to the allocation of hidden or blinded methods, the publication time and the study area were not limited. Our study patients included preterm infants with respiratory distress syndrome, and treatment with a stable, recommended dose of pulmonary surfactants was required before entry into the enrichment period. The control group was given conventional treatment, and the experimental group was given conventional treatment and pulmonary surfactants. In addition to interventions, other routine medical treatments were consistent between the two groups. The primary outcome of the analysis was the mortality rate of infants with NRDS. We excluded documents with incomplete data, in which the research design was defective or the statistical method was not correct, semi-randomised controlled trials, non-randomised controlled trials, observational studies, expert reviews, letters, and repeated publication studies.
Data extraction
Two researchers independently screened the literature, extracted the data, and cross-checked. If there was any disagreement, a third party was consulted to assist in the judgment. When reading the literature, the questions and abstracts were read first. After excluding the clearly unrelated documents, the full text was read to determine the final inclusion. The data extraction content includes: 1) Basic information for inclusion in the study, including first author and publication time. 2) The basic characteristics of the subjects, including the number of samples in each group, the average age of the patient, and the disease. 3) Intervention-specific details. 3) Key elements of bias risk assessment. 4) Outcome indicators and outcome measurement data of interest. Lack of information led to contacting the author to supplement the data as much as possible.
Risk of bias assessment
Two investigators evaluated the bias risk of inclusion in the study in accordance with the Corchrane Handbook for RCT bias risk assessment tools.
Statistical analysis
This study used a Bayesian grade model to perform a mesh meta-analysis of outcome measures using R software. The count data used the odds ratio (OR), and the interval estimate used 95% CI as the effect size indicator. P < 0.05 was set a statistically significant standard. If the p-value of Cochran’s Q test statistic was less than 0.05 or the I2 statistic is larger than 50%, then there was significant heterogeneity among included studies for each pairwise comparison. As a result, the fixed-effect model may not be appropriate for synthesising direct evidence, and the random-effects model should be used instead [9]. After comparing the various interventions, the ranking probability table was used to rank the pros and cons of the intervention (the value indicates the probability of the intervention at the nth position). R packages (coda, lattice, gemtc, rjags, igraph) were used to map the mesh of each intervention, presenting a direct and indirect comparison between interventions. A funnel chart was drawn to make a qualitative judgment on publication bias.
Results
Literature search results
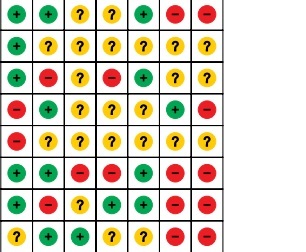
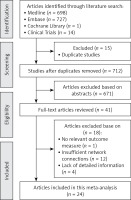
Figure 1 shows the results of the literature search. The published studies for pulmonary surfactant in the treatment of respiratory distress syndrome in preterm infants (up to January 2019) were retrieved from Medline (698), EMbase (727), the Cochrane Library (1), and Clinical Trials (14). By excluding duplicate (15) and unrelated literature records (671) and further reading the full text, we excluded studies with no relevant outcome measure, insufficient network connections, and lack of detailed information. Finally, 24 studies were used for the final data synthesis. Figure 2 shows the results of the risk of bias of 24 studies included in this meta-analysis [5, 10–32]. The characteristics of the included studies are shown in Table I. The pattern of evidence within the network is displayed in Figure 3.
Table I
Characteristics of included studies
Figure 2
Risk of bias of the included RCTs (Review authors’ judgments about each risk of bias item for each included study. +, low risk; −, high risk; ?, unclear risk)

Figure 3
Network of randomised controlled trials comparing different pulmonary surfactant for respiratory distress syndrome in the treatment of preterm infants. The thickness of the connecting lines represents the number of trials between each comparator, and the size of each node corresponds to the number of subjects who received the same pharmacological agent (sample size). (A: Survanta; B: Alveofact; C: Infasurf; D: Curosurf; E: Surfaxin; F: Exosurf)
Results of pairwise meta-analysis
The results of pairwise meta-analysis show that patients with the following drugs appeared to have significantly reduced mortality of respiratory distress syndrome compared with beractant: surfactant A (OR = 0.53, 95% CI: 0.31–0.90), calfactant (OR = 0.91, 95% CI: 0.85–0.97), poractant (OR = 0.72, 95% CI: 0.67–0.77), lucinactant (OR = 0.80, 95% CI: 0.71–0.90), and colfosceril (OR = 0.93, 95% CI: 0.87–0.99) (Table II). Moreover, there was no significant heterogeneity among studies for the above significant results (P-heterogeneity > 0.05 and I2 < 50%) (Table II).
Table II
Summary odds ratios of pulmonary surfactant and heterogeneity for each direct comparison
Network meta-analysis
Table III shows the results produced by network meta-analysis. Patients with the following drugs appeared to have significantly reduced mortality of respiratory distress syndrome compare with beractant: surfactant A (OR = 0.45, 95% CI: 0.23–0.87), calfactant (OR = 0.86, 95% CI: 0.77–0.96), poractant (OR = 0.71, 95% CI: 0.51–0.95), lucinactant (OR = 0.79, 95% CI: 0.66–0.93), and colfosceril (OR = 0.87, 95% CI: 0.76–0.98).
Table III
Network meta-analysis comparison
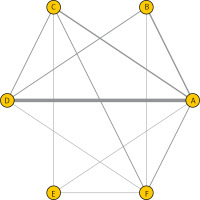
The corresponding results of SUCRA values are presented in Figure 4. The corresponding rankings based on SUCRA values are listed as: beractant (8.9%), surfactant A (93.8%), calfactant (40.3%), poractant (65.4%), lucinactant (59.8%), and colfosceril (31.6%). Surfactant A drugs appeared to have the best efficacy in reducing mortality of respiratory distress syndrome in preterm infants.
Figure 4
Surface under the cumulative ranking curve (SUCRA), expressed as percentages, ranking the therapeutic effects and safety of treatments for respiratory distress syndrome in preterm infants. For efficacy and safety assessment, the pharmacological agent with the highest SUCRA value would be the most efficacious and safe treatment (A: Survanta; B: Alveofact; C: Infasurf; D: Curosurf; E: Surfaxin; F: Exosurf)
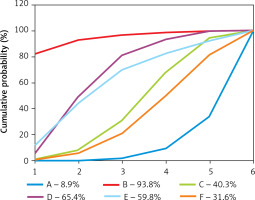
Publication bias
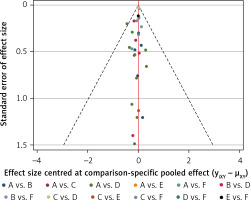
Figure 5 shows the results of publication bias. The red line suggests the null hypothesis that the study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates. No significant publication bias was observed.
Figure 5
Comparison-adjusted funnel plot for the network meta-analysis. The red line suggests the null hypothesis that the study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates. Different colours represent different comparisons (A: Survanta; B: Alveofact; C: Infasurf; D: Curosurf; E: Surfaxin; F: Exosurf)
Discussion
Progressive dyspnoea, increased heart rate, irritability, skin cyanosis, inspiratory depression, respiratory failure, and respiratory palsy are the main clinical manifestations of neonatal respiratory distress syndrome, and severe symptoms of organ failure occur in severe cases. If untreated, severe NRDS may lead to impaired pulmonary function and a ventilation/perfusion mismatch resulting in systemic hypoxaemia. The disease is more common in preterm infants [33]. The incidence of this disease is higher in neonates with gestational age less than 32 to 33 weeks. Due to the lack of pulmonary surfactant and immature lung tissue, pulmonary fluid transport disorder and progressive atelectasis are the basic characteristics of the disease [34].
Pulmonary surfactant is a mixture of specific proteins and phospholipid, which is widely distributed on the alveolar surface, and its main function is to effectively reduce the surface tension of the lung [35]. A number of randomised controlled trials have shown that exogenous PS replacement therapy can reduce the severity of respiratory failure and the incidence and mortality of NRDS [36, 37]. In 1990, the Food and Drug Administration (FDA) formally approved the use of PS for routine replacement therapy in children with NRDS. Exogenous PS replacement therapy has gradually become the standard therapy for premature infants with NRDS. Exogenous PS endotracheal intubation into the alveoli is the main method of treatment of NRDS. Early, adequate application can obtain obvious clinical results, shorten the course of disease, reduce the incidence of complications, reduce mortality, and improve the prognosis [38]. We performed a network meta-analysis based on RCT of NRDS treated with PS in order to further clarify the role of different exogenous PS.
By analysing the value of pulmonary surfactant therapy in NRDS, our study shows that pulmonary surfactants are more effective in reducing the mortality of respiratory distress syndrome in preterm infants compared with Survanta. Alveofact drugs appeared to be the most efficacious in reducing mortality of NRDS. The mechanism of exogenous PS in the treatment of NRDS, on the one hand, obviously reduces the alveolar surface tension of NRDS, increases alveolar compliance, increases lung volume and functional residual volume, and improves the ventilation function of the lung. On the other hand, pulmonary oedema, exosmosis of plasma content, formation of hyaline membrane of the lung, progressive dyspnoea, and respiratory failure occurred in children with increased pulmonary vascular permeability [39, 40]. Therefore, exogenous PS in the treatment of NRDS achieved good results, reducing complications and reducing mortality. Surfactant A is extracted from bovine lung lavage. Bovine lung lavage fluid is a common pulmonary surfactant derived from bovine lung tissue [41]. It can significantly reduce alveolar surfactant and increase lung compliance and oxygenation function. This product has a significant reduction in alveolar surfactant, which can increase lung compliance and oxygenation; however, it has a higher rate of pneumothorax. At the same time, the product has an obvious promotion effect on the secretion and synthesis of pulmonary surfactant, and it can effectively reduce the occurrence of alveolar collapse. In addition, bovine lung lavage fluid plays a significant role in reducing inflammatory response and in the treatment of various types of diseases such as bronchopulmonary dysplasia pneumonia pulmonary haemorrhage. It can be seen that this product has significant clinical value in the treatment of NRDS [42, 43].
A meta-analysis is a descriptive quadratic analysis, which has some defects. First, the sample size and basic treatment were different, and there was some heterogeneity among the indexes of observation and analysis and the adverse events. Second, although all included in the study were randomised and controlled, there were fewer double-blind and placebo-controlled trials, so the quality of study inclusion was low. Third, the results of analysis can be used as a reference for clinical application because all the observed indexes are mortality rate, which does not involve medium- and long-term curative effect. Fourth, most of these studies were not detailed in legal reports, such as the absence of a random allocation method, the implementation of the allocation concealment, or the implementation of the blind law, which leads to the existence of varying degrees of bias and risk.
In conclusion, our findings underscore the notion that, compared with beractant, other pulmonary surfactants are more effective in reducing mortality in NRDS. Surfactant A showed the be best efficacy in reducing the mortality of NRDS. However, due to the low quality of the inclusion study, this conclusion needs a large sample, which is further confirmed by the high-quality research.