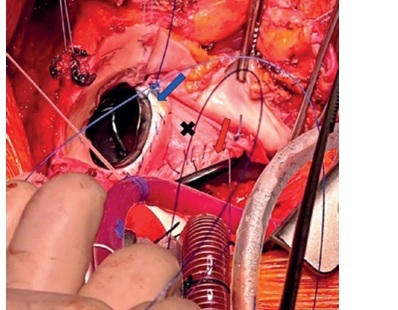
Subvalvular aortic stenosis (SAS) is a rare disease of pathological fibromuscular proliferation within the left ventricular outflow tract (LVOT) [1, 2], forming discrete or diffuse stenotic lesions and leading to a gradual manifestation of LVOT obstruction (LVOTO) [1–3]. Diffuse (tunnel-type) stenosis, in particular, has a progressive and highly recurrent natural history, and presents a more technically challenging surgery [1]. Aggressive resection is required for adequate relief of obstruction [2–5], and residual disease may lead to recurrent stenosis [5, 6].
The Commando procedure involves simultaneous aortic valve replacement (AVR), mitral valve replacement (MVR), and reconstruction of the aortomitral curtain (AMC), which may be performed concomitantly to myectomy. Outcomes of the Commando procedure have previously been reported for at least 1 case of SAS [7]. This article concerning the use of the Commando procedure in combination with myectomy for a severe recurrent case of diffuse SAS: (1) reports early clinical outcomes, (2) discusses the significance of strategic LVOT reconstruction in the modification of the hemodynamic and geometric factors involved in pathogenesis of recurrent SAS, and (3) examines the role of the Commando procedure in diffuse SAS.
A 26-year-old female presented with NYHA II–III symptoms from recurrent SAS with complications of severe aortic regurgitation (AR), moderate mitral stenosis (MS) and mild mitral regurgitation (MR). This disease was associated with a history of congenital atrioventricular septal defect (AVSD), which was surgically closed at the age of 18 months; in the following 10 years she developed her index SAS and AR for which she underwent surgical resection and aortic valve repair at the age of 12 years.
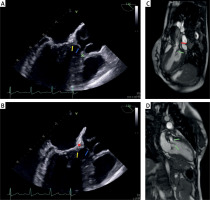
Multimodal imaging (Figure 1) revealed a diffuse tunnel-type stenosis (mean LVOT diameter = 1.6 cm) along with a discrete membranous lesion, resulting in LVOTO with gradients of 136/91 mm Hg (peak/mean) and maximum flow velocity (Vmax) of 5.8 m/s. Fibromuscular lesions extended across the heavily thickened AMC and anterior mitral leaflet (AML), and merged into the base of the aortic valve (AV) cusps, leading to limited mobility and coaptation defects. AR fraction was 50% with holodiastolic flow reversal, MR fraction 30%, mitral gradient of 14.7/7.7 mm Hg, and mitral valve (MV) area of 1.6 cm2. The interventricular septum (IVS) was mildly hypertrophic with postoperative morphologic changes and a thickness (IVSd) of 1.3 cm.
Figure 1
A, B – Transesophageal echocardiography, long-axis view: membranous and tunnel-like structures (blue and yellow arrows, respectively) with involvement of the base of the right coronary cusp (green cross) and the intervalvular fibrosa (red cross), C, D – Cardiac magnetic resonance: subaortic membrane (green arrows) with features of accelerated blood flow (red arrow)
The stenosis was severe [1], and necessitated very aggressive resection for adequate relief to an acceptable postoperative gradient. Due to the heavily thickened AMC forming the bulk of the obstruction, this warranted full-thickness resection of the AMC in addition to the planned septal myectomy and AVR. Due to the patient’s wishes for a sustained durable period of freedom from reintervention in her early/middle age, and the limited possibilities of future reinterventions, definitive management of MV disease was also a priority. For this reason, elective MVR was proposed at the time of concomitant surgery. The (patient-specific) risk of SAS recurrence is high. Through reconstruction of the fibrous skeleton (the Commando procedure), annular enlargement and “posterior LVOT-plasty” could be performed to maximally unload pressure gradients, correct turbulent flow, and mitigate shear stress injury. Ultimately, by surgical modification of the hemodynamic and geometric factors involved in the pathogenesis of fibromuscular proliferation (and with wider diameters better tolerating recurrent stenosis), this strategy could potentially delay or avoid the need for reoperation.
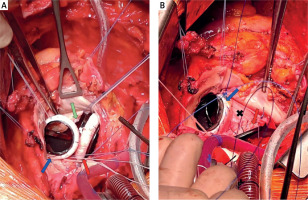
A cautious but otherwise standard sternotomy was performed and, due to past surgical history, cardiopulmonary bypass was established with peripheral cannulation. The AV and MV were exposed simultaneously through a continuous incision opening into the aorta and left atrium. Both native valves were excised. Intraoperative findings reported more severe pathologic changes that extended beyond the AMC and necessitated a wider resection of the intervalvular fibrous skeleton than was anticipated. Through the common left ventricular orifice (Figure 2 A), myectomy of the IVS was performed. 25 mm mitral and 21 mm aortic On-X (mechanical) valves were implanted into their remaining hemiannuli (Figure 2 B), and the fibrous skeleton was reconstructed around the prosthetic valves using a tailored patch of bovine pericardium. Upon this structure, a second patch of pericardium was used to reconstruct the left atrium and the aortic root. Cardiopulmonary bypass time was 294 min and cross-clamp time was 140 min. The patient was weaned off cardiopulmonary bypass without incident.
Figure 2
A – Intraoperative view showing the common ventricular orifice (green arrow) with the aortic and mitral prosthetic valves (blue and red arrows, respectively), B – reconstructed fibrous skeleton (black cross)
Two units of blood products were transfused postoperatively. No other operative morbidities were reported. There were no conduction disturbances. The patient was extubated within 12 h and discharged from intensive care within 24 h. The combined LVOT-AV gradient was 19/12 mm Hg. AV area was 2.4 cm2 (1.39 cm2/m2). The patient was in NYHA I–II function class at discharge on postoperative day 8. Trivial pleural effusion was noted on 2-week follow-up; the first four weekly follow-ups were otherwise unremarkable.
Progressive SAS results in increasingly detrimental consequences of AV injury and ventricular hypertrophy [1, 6], and higher preoperative and/or postoperative gradients have been associated with worse outcomes (recurrence/reoperation) [1–3, 6, 8–10]. Based on these findings, authors have suggested that early intervention [6] and/or complete relief of obstruction [1–3, 5, 6, 11, 12] may improve outcomes. The Konno procedures (Konno-Rastan or modified Konno) provide effective relief of LVOTO with generally favorable outcomes [3, 5, 6, 11–14], and are often considered (in various contexts) to be the definitive surgical option [1–3, 5, 11, 12] if not the treatment of choice [5]. In combination with full-thickness myectomy, the anterior septoplasty allows for maximal enlargement of the LVOT [2, 3], which is believed to lower risk of recurrence or reoperation [2, 3, 6, 11, 12]. However, current understanding of the causal relationship between LVOT enlargement and recurrence risk is incomplete [2, 3]. Some have suggested that, in addition to longer-lasting relief of LVOTO, surgical remodeling of the LVOT may have an additional disease-modifying role by inhibiting recurrent pathological changes [2, 3].
Several limitations must be noted. Both Konno procedures and the Commando procedure are very infrequently performed surgeries. Short- and long-term outcomes of the Commando procedure for SAS are still relatively unknown. Evidence supporting the use of the Konno procedures is, in large part, limited to either single-arm studies [3, 5, 6, 11, 12–15] or from comparison between imperfectly matched groups. While its (evidence-based) role of effective LVOTO relief is well-described, the (largely concept-based) disease-modifying role is rarely discussed or considered in the literature. Lastly, a recent study reported that despite early alleviation, recurrence occurs over time after the Konno procedures [15].
In conclusion, the Commando procedure was performed for concomitant and definitive management of AV, MV and AMC disease. The heavily thickened AMC was treated with full-thickness resection, followed by patch reconstruction of the iatrogenic defect. This surgery demonstrated the use of the Commando (posterior LVOT-plasty) procedure in a similar role to the Konno (anterior LVOT-plasty) procedure. Early hemodynamic outcomes were relatively favorable (relative to the high preoperative gradients) and grossly comparable to that of other studies for both the Commando [7] and Konno [3, 5, 6, 10–15] procedures (although these results must be compared and interpreted with caution). Although current findings are supportive of this novel role of the Commando procedure, our collective experience is still very limited in the literature. Also limited is the study of the proposed disease-modifying role, as has been described for the modified Konno procedure [2]. Various studies have identified the hemodynamic and geometric factors associated with recurrence of SAS [2, 3]. Rationally, surgical modification of these pathophysiological factors is promising of considerable benefit. Long-term large-volume studies will be needed to evaluate this; even then, causality is difficult to prove [3].