Introduction
Plasmablastic lymphoma (PBL) is an extremely rare and distinct subtype of B-cell non-Hodgkin lymphoma (NHL) [1, 2]. PBL exhibits mixed morphological characteristics of diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM) with its highly aggressive course and plasmacytic differentiation [3]. The cell origin of PBL is considered the post-germinal center B-lymphocyte or plasmablast. Although PBL cells usually express classic biomarkers of plasmacytic differentiation (CD38, CD138 and MUM1) such as MM and seldomly express B-lymphocyte differentiation markers (CD19, CD20, and PAX5), PBL bears a striking resemblance to DLBCL via genomic profiling [4]. It is often believed that PBL afflicts immunocompromised individuals, including those infected with human immunodeficiency virus (HIV), those receiving intense chemotherapy or radiotherapy for cancers, those undergoing organ or stem cell transplantation and those receiving immunosuppressive drugs [5–7]. Plasmablastic lymphoma was once regarded as a tumor that predominantly arose in patients with HIV-infection [8]. However, Castillo et al. reported 71 HIV-negative PBL patients with unique clinicopathological characteristics markedly distinct from their HIV-positive counterparts [9].
Plasmablastic lymphoma is notorious for resistance to chemotherapy, invasiveness and rapid progression. The CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) serves as the first-line choice for the treatment of NHL. However, the National Comprehensive Cancer Network (NCCN) guidelines stipulate that standard CHOP seems ‘inadequate and less intense’ for PBL [10]. Despite the use of various regimens, the prognosis of PBL remains bleak. Owing to the rarity of PBL, previous publications mainly focus on clinical case reports or case series with a small sample size. Little is known about this rare hematological malignancy. What is worse, neither a standardized regimen nor a consensus for PBL treatment has been established yet. The objective of our study is to further explore the clinical characteristics, prognostic factors and therapeutic modality of PBL on a larger scale. Patients in our study derived from the Surveillance, Epidemiology, and End Results (SEER) database and our institution. We also discussed the recent therapeutic approaches and advances in PBL.
Material and methods
Patient selection
Initially constructed by the National Cancer Institute of United States (US), the SEER database accounts for nearly 30% of the US population across 18 cancer registries and is completely available to the public via formal application. We obtained authorization to have access to the unidentified individual information. According to the International Classification of Disease for Oncology, 3rd edition (ICD-O-3), we retrieved the SEER database for PBL patients from 2008 to 2016 using the “9735/3: plasmablastic lymphoma” histology code in the “site and morphology ICD-O-3 histology/behavior, malignant” field. The patient data were downloaded using SEER*Stat Software (Version 8.3.6, http://www.seer.cancer.gov/seerstat).
The patient data include age at diagnosis, gender, race, primary anatomical site, Ann Arbor stage, previous malignancy history, survival months, vital status, cause of death and the first round of treatment modalities (chemotherapy or not, radiation or not). However, the detailed HIV infection status, immunohistochemical results, chemotherapy regimens, types of surgery, drug dosage and radiation dosage were not recorded in the SEER database. Patients without pathologically confirmed diagnosis or sufficient follow-up information were excluded.
Statistical analysis
Overall survival (OS) was determined from the time of diagnosis to death from any cause or the last follow-up. Disease-specific survival (DSS) was calculated from diagnosis to the date of death caused by PBL. Kaplan-Meier curves and the log-rank test for OS and DSS were conducted to compare each potential variable related to the prognosis. Variables with a p-value < 0.1 in the univariate Cox regression model were incorporated into the multivariate Cox model to determine the independent prognostic factors, with a hazard ratio (HR) > 1 indicating adverse factors. All tests were two-sided, with a p-value < 0.05 regarded as statistically significant. All statistical analyses were performed with SPSS Software (Version 26.0, IBM Corp., USA).
Patient group from our institution
We then retrospectively identified 21 PBL patients in our institution between Aug 2009 and Aug 2018. In accordance with the 2008 WHO classification of tumors of hematopoietic and lymphoid tissues, the pathological diagnosis was made through hematoxylin-eosin staining and immunophenotyping results.
Relevant clinical information of 21 PBL patients included age at diagnosis, gender, HIV infectious status, Eastern Cooperative Oncology Group performance status (ECOG-PS), Ann Arbor stage, international prognostic index (IPI), primary tumor locations, immunochemistry results, specific therapeutic regimen, the best response to therapy and the final survival outcomes. Immunohistochemical studies were conducted on formalin-fixed, paraffin-embedded tissue sections by utilizing antibodies including CD138, CD38, CD10, CD20, CD79, PAX5, MUM1, CD30, CD56, BCL6, CD5, CD3, CD45 and Ki67. In situ hybridization (ISH) for EB virus-encoded small RNA (EBER) was also detected by employing a fluorescein-labeled peptide nucleic acid probe on the tissue sections.
Given the rarity of PBL and the small sample size from our institution, we consider it unreasonable to conduct a log-rank test or Cox regression analysis. Therefore, we only plotted an OS curve to demonstrate the patient survival outcomes.
Results
Patients from the SEER population registries
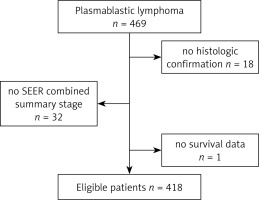
After rigorous identification, a total of 418 eligible patients were ultimately enrolled in our study. The flow diagram of the selection process is presented in Figure 1. The demographic and clinical characteristics of PBL patients are summarized in Table I. The median age at diagnosis was 56 years. PBL has a striking gender predilection for males, with the ratio of male to female patients being 3.5 : 1. Patients with B symptoms accounted for 23.68%. Nearly 18.7% of the patients had a previous history of other malignancies. However, the SEER database did not record the information on the HIV infection status. Only 6% of the patients received both chemotherapy and radiation at the initial diagnosis, while 66% received chemotherapy alone and 26.8% of patients were untreated.
Table I
Demographic and clinical characteristics of 418 patients from SEER database
The distribution of primary anatomical sites is presented in Table II. Of all the patients, 185 (44.26%) cases were detected within the lymph nodes and 233 (55.74%) cases suffered from extranodal infiltration. The most frequently involved extranodal site was the gastrointestinal tract (20.10%), followed by nasal cavity/paranasal sinuses (11.72%) and oral cavity, mouth and tongue (5.50%).
Table II
Distribution of primary anatomic sites of plasmablastic lymphoma
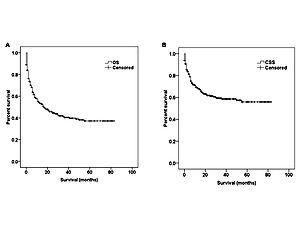
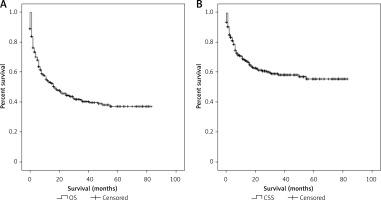
Some patients died promptly after the initial diagnosis due to the high aggressiveness and rapid progression of PBL. Therefore the follow-up time is very short. Other patients survived several years after the diagnosis. The follow-up time of patients varied considerably. As was revealed in the Kaplan-Meier curves of OS (Figure 2 A) and DSS (Figure 2 B), the median OS for all PBL patients was 17 months (95% CI: 10.3–23.7). The 1-year, 3-year and 5-year OS rates were 54.4%, 40.4% and 37.2%, respectively. The median DSS was not reached, and the 1-year, 3-year and 5-year DSS rates were 69%, 58.6% and 55%, respectively.
Figure 2
Kaplan-Meier curves of the whole eligible cohort: A – overall survival, B – disease-specific survival
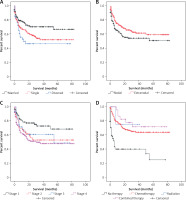
In the univariate assessment, marital status (p = 0.015), previous malignancy history (p = 0.003), primary site (p = 0.009), Ann Arbor stage (p = 0.001), therapeutic modality (p < 0.001) are the possible predictive factors of OS (Table III). Age, gender and race were not found to influence PBL prognosis. The predictors of DSS were similar to those of OS (Table III). Kaplan-Meier survival curves gave a vivid description of the association between various factors and OS (Figure 3) and DSS (Figure 4) of PBL patients. As revealed in Table IV, multivariate analysis verified that previous malignancy history, Ann Arbor stage and therapeutic modality were independent prognostic factors. Patients who suffered from previous malignancy had a significant survival disadvantage (HR = 1.444, 95% CI: 1.037–2.001) compared to those without previous cancer. Patients with a higher Ann Arbor stage at diagnosis were at higher risk of death than those with a lower stage. In terms of therapeutic modalities, chemotherapy alone or chemotherapy combined with radiotherapy could significantly reduce the risk of death and extend the patients’ survival, yielding HR of 0.209 (95% CI: 0.152–0.288, p < 0.001) and 0.187 (95% CI: 0.089–0.394, p < 0.001), respectively. However, radiation alone without chemotherapy seemed useless.
Table III
Univariate Cox regression analysis of overall survival and disease-specific survival
Table IV
Multivariate Cox regression analysis of overall survival and disease-specific survival
Patients from our institution
We enrolled altogether 21 patients including 12 males and 9 females. The median age at diagnosis was 52 years. Clinical features and immunophenotypical results of patients from our institution are presented in Table V. All patients were HIV-negative and were not in immunocompromised status. The tumor arose extranodally in 16 (76.2%) cases. Plasma cell marker positivity including CD38, CD138, and MUM1 was universally detected (81%, 90% and 85.7%, respectively), while CD20 and PAX5 negativity was detected in most cases (90.5% and 86.7%, respectively). The proliferation index Ki-67 was commonly high, ranging from 40% to 95% with 16 cases > 80%. EBER positivity was detected in 5 out of 17 cases (29.4%).
Table V
Clinical features and immunophenotypical results of 21 patients with plasmablastic lymphoma in our institution
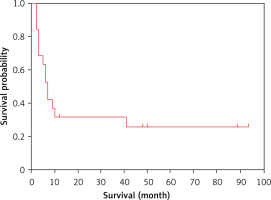
Detailed information on therapeutic regimen and survival outcomes is summarized in Table VI. The Kaplan-Meier plot for the whole group revealed that median OS was 7 months (Figure 5). The CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOPE regimen (CHOP + etoposide) was selected as the first-line therapy for most patients. DA-EPOCH (dose-adjusted etoposide + doxorubicin + vincristine + cyclophosphamide + prednisone), DHAP (cisplatin + high-dose cytarabine + dexamethasone), DICE (cisplatin + ifosfamide + etoposide + dexamethasone), ESHAP (etoposide + methylprednisolone + high-dose cytarabine + cisplatin), GDP (gemcitabine + cisplatin + dexamethasone) and HyperCVAD/high-dose MTX/Ara-C (hyper-fractionated cyclophosphamide + vincristine + doxorubicin + dexamethasone alternating with methotrexate and cytarabine) were reasonable options. Regimens for the treatment of MM such as PAD (bortezomib + doxorubicin + dexamethasone), PCD (bortezomib + cyclophosphamide + dexamethasone), TAD (thalidomide + doxorubicin + dexamethasone), and MPT (melphalan + prednisone + thalidomide) were also administered. Only 1 patient who received autologous stem cell transplantation (ASCT) with thalidomide maintenance was still alive at the end of our study. A complete response (CR) was achieved in only 3 patients who were still alive at the study endpoint. A partial response (PR) was obtained in 10 patients. Efficacy was limited and transient in most patients. Fourteen patients later died due to disease progression.
Table VI
Therapeutic regimen and survival outcomes of 19 treated patients with plasmablastic lymphoma in our institution
[i] ASCT – autologous stem cell transplantation, CAD – cyclophosphamide + doxorubicin + dexamethasone, CDOP – cyclophosphamide + liposomal doxorubicin + vincristine + prednisone, CHOP – cyclophosphamide + doxorubicin + vincristine + prednisone, CHOPE – cyclophosphamide + doxorubicin + vincristine + prednisone + etoposide, CVE – cyclophosphamide + vincristine + etoposide, DA-EPOCH – dose-adjusted etoposide + doxorubicin + vincristine + cyclophosphamide + prednisone, DHAP – cisplatin + high-dose cytarabine + dexamethasone, DICE – cisplatin + ifosfamide + etoposide + dexamethasone, ESHAP – etoposide + methylprednisolone + high-dose cytarabine + cisplatin, GDP – gemcitabine + cisplatin + dexamethasone, HyperCVAD – cyclophosphamide + doxorubicin + vincristine + dexamethasone, MINE – mitoxantrone + ifosfamide + etoposide, MOPE – mitoxantrone + vincristine + prednisone + etoposide, MPT – melphalan + prednisone + thalidomide, PAD – bortezomib + doxorubicin + dexamethasone, PCD – bortezomib + cyclophosphamide + dexamethasone, TAD – thalidomide + doxorubicin + dexamethasone, Thal – thalidomide, VAD – vincristine + doxorubicin + dexamethasone, CR – complete response, PD – progressive disease, PR – partial response, NA – not available.
Discussion
Initially described in the oral cavity of HIV-infected patients in 1997, PBL is characterized by male predominance, predilection for extranodal involvement and higher incidence among immunocompromised patients [8]. Highly active anti-retroviral therapy (HAART) is oriented to kill HIV, which mainly includes stavudine in combination with lamivudine and nevirapine, zidovudine in combination with lamivudine and efavirenz, and emtricitabine in combination with tenofovir and Kaletra [11, 12]. Some case reports depicted the spontaneous regression of HIV-related PBL after use of HAART. However, a sustained CR could not be achieved and patients experienced early relapse [13, 14]. A study from the LYmphoma Study Association (LYSA) Group also verified that HAART alone without chemotherapy could not achieve a sustained CR [15]. The administration of HAART combined with chemotherapy is preferably recommended in HIV-positive patients.
With the increased understanding of PBL, we have come to realize that PBL also occurs in HIV-negative or other immunocompetent patients. All patients from our institution were HIV-negative and previously immunocompetent with a normal CD4+T cell count. HIV-positive and HIV-negative PBL are two strikingly distinct subentities with different clinicopathological characteristics. HIV-negative patients bore a bleaker prognosis, worse response to chemotherapy and shorter OS than HIV-positive patients [9, 15, 16]. Some cases with oral cavity involvement are associated with EB virus (EBV). The criteria for PBL diagnosis have varied over time. Some researchers rigorously defined coexistence of a lesion in the oral cavity and presence of HIV or EBV. But now the classification of PBL has been defined in a broader sense, in which morphology and immunophenotype correspond to B immunoblasts or plasma cells with EBV-negativity and extranodal involvement in other parts of the body [17].
We should make a reasonable differential diagnosis to tell apart anaplastic lymphoma kinase-positive large B-cell lymphoma (ALK+ LBCL), primary effusion lymphoma (PEL), Epstein-Barr virus-positive DLBCL, human herpes virus 8-positive DLBCL, intravascular large B-cell lymphoma (IVLBCL) and other specific variants of DLBCL [2, 18]. PBL is not always associated with EBV positivity. PBL exhibits a ‘terminal B-cell differentiation’ phenotypical feature with upregulation of plasma cell markers (CD138, CD38) and downregulation of B-cell markers. ALK-positive large B-cell lymphoma (ALK + LBCL) exhibits immunoblast-like or plasmablast-like features with prominent overexpression of ALK protein due to ALK translocation and prevalent positivity of plasma cell-associated markers [19, 20].
There has been no standard therapeutic regimen for PBL. Arora’s et al. study reported that median OS of the entire cohort was 15.9 months. The median OS for treated and untreated patients was 17.9 months and 0.9 months, respectively [21]. Despite the fact that the most commonly used regimen is CHOP or CHOP-like at present, the NCCN guidelines state that CHOP is ‘inadequate and inefficient’ and propose more intensive regimens including DA-EPOCH, HyperCVAD and CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) [10]. The MD Anderson center reported that patients who underwent the CHOP regimen tended to enjoy better OS compared with those who underwent the hyper-CVAD regimen (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), which can be explained by the fact that hyper-CVAD is much more intense and possesses more “toxicity” than the CHOP regimen [22]. The efficacy of adding rituximab (a kind of anti-CD20 monoclonal antibody) to conventional chemotherapy remains obscure. We speculate that the addition of rituximab is unlikely to achieve clinical benefit as PBL cells scarcely express CD20 antigen. Several studies have shown that EPOCH displayed better efficacy and survival outcomes than CHOP, especially in highly aggressive B-cell NHL [23, 24]. Castillo et al. reported that 3 PBL patients achieved a durable CR to V-DAEPOCH (bortezomib in combination with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) with OS of 12, 18, 24 months respectively [25]. Dittus et al. reported that the V-DAEPOCH regimen was efficacious and well tolerated in 8 PBL patients, achieving a CR rate of 100% and 2-year OS rate of 50% [26]. In 2018, Castillo et al. reported that among 16 patients who received the V-DAEPOCH regimen (range: 4–6 cycles), CR was observed in 15 patients and PR in 1 patient. The 5-year OS rate was 65%. Of the 5 dead patients, 3 died due to PBL progression and 2 died of infection [27]. The infusional EPOCH regimen resulted in long-lasting remission in 13 patients with extranodal involvement [28]. The above observations indicate that V-DAEPOCH is an effective and frontline therapeutic regimen for PBL.
Lenalidomide is a kind of immunomodulatory agent, which is widely used for MM treatment. The RCD regimen (lenalidomide in combination with cyclophosphamide and dexamethasone) was reported to successfully treat a stage IE HIV-negative PBL patient, achieving an at least 24 months’ durable CR [29]. Similarly, an HIV-positive PBL was effectively treated with lenalidomide combined with CHOP [30].
A case-control study suggested that there was no significant difference in non-relapse mortality, 2-year disease-free survival and OS between HIV-positive and HIV-negative NHL patients who received ASCT. That is to say, HIV status does not influence the long-term outcome of ASCT for NHL [31]. Autologous stem cell transplantation during first CR after intensive induction therapy is a feasible solution to improve the PBL outcome [32]. Infusional EPOCH with subsequent consolidation ASCT in the eligible patients was recommended for treating HIV-associated PBL [33]. The European Society for Blood and Marrow Transplantation registry reported 24 PBL patients who underwent ASCT. Those who were autografted in CR showed a significantly decreased relapse risk and overall mortality risk [34]. ASCT warrants to be further investigated as first-line consolidation and salvage therapy for both HIV-positive and negative PBL patients especially in the absence of effective therapeutic options [35]. Nishi et al. reported that an HIV-negative woman with chemotherapy-refractory PBL had disease-free survival of more than 18 months after umbilical cord stem cell transplantation (UBSCT) [36].
PRDM1 gene mutation was frequently found in PBL and greatly augmented the oncogenicity of the MYC gene to enhance the proliferative activity by inducing MYC translocation or amplification. In a sense, PRDM1 mutations can be genetically regarded as a second hit in PBL and may become a therapeutic target in the future [37]. A recent study found that CD30 expression was pronounced in PBL tissue and will become a target with the potential use of brentuximab vedotin [38].
There were several inevitable defects in our study mainly owing to the inherent drawbacks of the SEER database. Firstly, the SEER registry is a population-based registry and does not record some important individual information, such as ECOG-PS, IPI, tumor size, immunohistochemistry results, FISH results, cytogenetic abnormalities, virus infection status and lactate dehydrogenase (LDH). Secondly, information concerning comorbidities, disease progression and relapse was not documented. Thirdly, the specific drug dosage and radiation dose were not recorded in the SEER database. Whether PBL patients received ASCT was unknown from the SEER. What is more, dates of information retrieval spanned a long period of time, which witnessed the variations in diagnostic criteria and the advancement of therapeutic approaches. The high heterogeneity of PBL cannot be neglected as well. Lastly, research on patients from our institution involved a small sample size due to the scarcity of PBL.
In conclusion, PBL is an extremely rare and aggressive entity of B-cell NHL. Despite being initially reported in HIV-infected patients, PBL has also been identified in other immunocompetent patients. PBL has a male predilection. The cohort from the SEER database revealed that previous malignancy history, Ann Arbor stage, and therapeutic modality were independent prognostic factors. Bortezomib combined with DA-EPOCH may serve as an effective and frontline therapeutic regimen for PBL. Prospective clinical trials should also be further conducted to explore the efficacy and safety of novel agents including chimeric antigen receptor T cell (CAR-T) therapy, immune checkpoint inhibitors and other monoclonal antibodies for the treatment of PBL.