Introduction
Transcatheter aortic valve implantation (TAVI) represents a real revolution in the field of interventional cardiology for the treatment of elderly or high-risk surgical patients with severe symptomatic aortic valve stenosis (SAS) [1]. Today, TAVI seems to play a key and a reliable role in the treatment of intermediate and maybe low-risk patients with SAS [2]. TAVI has also evolved from a complex and hazardous procedure into an effective and safe therapy by the development of new generation devices [3].
Myval, a newer-generation balloon-expandable (BE) transcatheter heart valve (THV) system, is designed on a nickel-cobalt alloy (MP35N) frame – which enables optimal radial strength and radiopacity – and decellularized bovine pericardium tissue, crafted into a tri-leaflet valve [4]. The valve consists of a novel hybrid honeycomb scaffold design. The upper part of the frame is composed of a single row of tall, large, and open-cell configuration to ensure unjailing of the coronary ostia that preserves coronary flow; the lower part of the frame is composed of two short rows of tightly packed, close-cell hexagonal configuration providing high radial strength required at the annular base. This unique pattern of Myval helps the operator in planning the precise placement of the valve and ensures its orthotopic deployment [4–6]. The lower closed cells of the Myval THV are covered externally with a sealing cuff, made of polyethylene terephthalate, to form an external buffing that minimizes or eliminates paravalvular leak (PVL). The THVs were available in different sizes: conventional (20, 23, 26, and 29 mm), medium (21.5, 24.5, and 27.5 mm) and extra-large (30.5 and 32 mm; extra-large sizes). All sizes are compatible with 14-Fr expandable sheaths. The Myval is mounted over the balloon outside the patient. The Navigator balloon-expandable THV delivery system has a unique design featuring a proximal deep flexion handle and a distal balloon with two counter-opposing soft stoppers within that create a superficial low-profile crimping zone and thus a comfortable fit that prevents any unwanted movement of the Myval THV during crossover through the sheath or thereafter. The delivery system allows for flexion of the distal catheter system that ensures trauma-free negotiation across the aortic arch and minimizes or eliminates the risk of a periprocedural stroke during arch navigation [4–6]. Compared to old generation BE THV systems, the Myval has several advantages: First, the Myval THV has its own intermediate and extra-large sizes and all sizes can be used through a 14F Python sheath. Second, the valve is directly mounted on a balloon (similar to a stent) and can be inserted into the introducer sheath with a “just deliver and implant” approach. Third, the valve can be taken out through the introducer sheath and can be both reinserted and reimplanted [4–6].
Several observational studies and medical device registries around the world are already available, sharing data and experiences, methodologies and algorithms for sizing and implantation. Most of these sources of information are based on the criteria set by the Valve Academic Research Consortium (VARC) consortium. However, the rapid evolution of the field, including the expanding clinical indications, and novel therapy strategies have mandated further refinement and expansion of VARC criteria to ensure clinical relevance [7]. In 2021, the VARC published a revised document that provides an update of the most appropriate clinical endpoint definitions to be used when conducting transcatheter and surgical aortic valve clinical research. This multi-disciplinary approach has resulted in important suggestions for the methodologies applied in clinical research, updated endpoint definitions, as well as for reporting data from registries, observational studies, and trials [7].
TAVI is now considered an established treatment for high and intermediate surgical risk patients with SAS. However, limited data exist on long-term follow-up based on VARC-3 criteria. Limited data also exist on long-term follow-up of degenerative SAS patients who underwent TAVI with a new generation, balloon expandable Myval THV. Thus, the aim of this study was to investigate the performance and 2-year clinical outcome of the Myval THV system based on VARC-3 criteria among patients with SAS.
Material and methods
Design
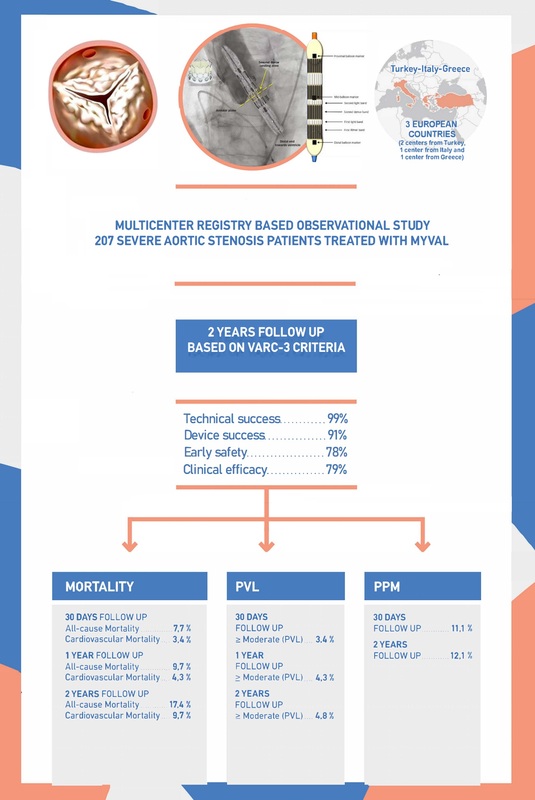
It was a multi-centre, registry-based, cohort study conducted from 2019 to 2021 in two centres in Turkey (Kocaeli University Medical Faculty, Kocaeli and Trabzon Ahi Evren Cardiovascular and Thoracic Surgery Training and Research Hospital, Trabzon), in one center in Italy (IRCCSOspedale Galeazzi Sant’Ambrogio, Milan) and in one center in Greece (Inter-Balkan Medical Center, Thessaloniki) to record procedural characteristics and clinical outcomes of TAVI with a new generation BE THV, the Myval (Meril Life Sciences Pvt. Ltd., Vapi, Gujarat, India).
Sample
The study included 207 patients (81 ±7 years old, age range 59 to 98 years, 94 [45%] men) who presented at the selected centres, with evidently degenerative SAS diagnosis (ICD-10, I35.0). Of them, 128 patients were from Turkey (80 ±7 years, 52 men), 58 were from Italy (83 ±5 years, 29 men) and 21 were from Greece (80 ±5 years, 13 men).
Setting
All consecutive SAS patients, of any age, who presented to the hospitals for TAVI with the Myval, and had a complete 2-year follow-up, were enrolled in the study. Patients were advised to undergo TAVI only if they were at high or intermediate risk for surgical aortic valve replacement (SAVR). Patients’ characteristics were recorded at four time points: at baseline, as well as at three follow-up examinations at 30 days, 1 year, and 2 years after implantation. A common standardised protocol was used for retrieving the information according to the VARC-3 criteria [7].
Bioethics
The study was approved by the local ethical committees of each participating hospital and complied with the Declaration of Helsinki principles (1989). All patients were informed by a physician of the study about their health condition, the available options, and risks, and the specific TAVI procedure. Moreover, patients were also informed about the aims of this registry-based study and provided written informed consent with agreement to participate.
Procedures
All patients underwent TAVI with the Myval THV, which is available in various sizes (traditional, 20, 23, 26 and 29 mm, intermediate, 21.5, 24.5 and 27.5 mm and extra-large, 30.5 and 32 mm).
Measurements
Socio-demographic, lifestyle and clinical measurements included, age, sex, anthropometric characteristics (body weight and height, for the calculation of body mass index (BMI) in kg/m2 and body surface area (BSA) in m2), smoking habits (recorded as current, ever, never smoker), personal medical history of hypertension, type 2 diabetes mellitus, hyperlipidaemia, previous coronary artery disease (CAD) or any other manifestation of CVD and family history of CAD. Procedural outcomes included pre-procedural imaging tools utilised by two dimensional transthoracic echocardiography (2D-Echo), and transoesophageal echocardiography (TEE). Electrocardiogram gated multislice computed tomography (CT) scan imaging was also applied to confirm the anatomy and the morphological type of the valve. Sizing in the balloon expanding valve was based on CT derived annulus area. The Society for Thoracic Surgery (STS) score [8], as well as the logistic EuroSCORE and EuroSCORE II [9], both validated risk-assessment tools for open heart surgery, were calculated. A predicted risk of 4–8% is considered intermediate risk of surgical mortality, and 8% or greater is considered high risk [8, 9]. The Katz Fragility Index was also calculated to assess the functional status of the patients and their ability to perform activities of daily living independently (theoretical range 0-6; higher values indicate better function) [10].
Endpoint(s)
The primary endpoint measure of the study was all-cause mortality, which includes cardiovascular and non-cardiovascular mortality during the 2-year follow-up period. Death meeting one of the following criteria was attributed as cardiovascular mortality: a) Related to heart failure, cardiogenic shock, bioprosthetic valve dysfunction, myocardial infarction, stroke, thromboembolism, bleeding, tamponade, vascular complication, arrhythmia or conduction system disturbances, cardiovascular infection (e.g. mediastinitis, endocarditis), or other clear cardiovascular cause, b) intraprocedural death, c) sudden death d) death of unknown cause. Death clearly related to a non-cardiovascular cause, such as respiratory failure not related to heart failure (e.g. pneumonia), renal failure, liver failure, infection (e.g. urosepsis), cancer, trauma, and suicide, was classified as noncardiovascular mortality [7].
Timing of mortality was classified as periprocedural, early mortality and late mortality according to VARC3 criteria [7]. Periprocedural mortality was defined as death meeting one of the following criteria: a) Occurring ≤ 30 days after the index procedure b) Occurring > 30 days but during the index hospitalization. Death occurring > 30 days but ≤ 1 year after the index hospitalization was classified as early mortality. Late mortality was defined as death occurring > 1 year after the index hospitalization [7].
Composite endpoints of the study within a period of 2 years include: technical success, device success, early safety and clinical efficacy. All these composite endpoints were clearly defined according to the VARC3 criteria [7].
Secondary endpoints, within a 2-year period, include: paravalvular leak (PVL) (the severity of PVL was categorised based on the sum of the circumferential lengths of each regurgitant jet vena contracta/the circumference of the outer edge of the transcatheter valve; acute myocardial infarction, stroke (disabling and non-disabling), life-threatening or disabling bleeding, acute kidney injury (stage 2 or 3), major vascular complications, and or conduction system disturbances resulting in a new permanent pacemaker implantation (PPI), and functional changes from baseline. The echocardiographic endpoints include mean aortic valve gradient, peak aortic valve gradient, peak aortic velocity, transvalvular, paravalvular, and total aortic regurgitation, and left ventricular ejection fraction (LVEF). All endpoints were defined according to VARC-3 criteria [7]. VARC-3 recommends the use of clinically relevant endpoints with consistent definitions, appropriate to the size and type of clinical studies [7].
Statistical analysis
Data were analysed using Stata version 17 statistical software (StataCorp, College Station, TX, USA). The data were collected and analysed from the three centres in a single analysis, as no statistical power was achieved to analyse data by centre. Categorical variables were expressed as absolute numbers and percentages, whereas continuous variables were expressed as mean (standard deviation – SD) or median (25th to 75th interquartile range – IQR), as appropriate according to their distribution. Time to event during the follow-up period was recorded monthly. Generalised linear models for repeated measurements, with various trends (i.e., linear, quadratic, 3rd order), were applied to evaluate progression of LVEF and mean aortic valve gradient of the patients. Logistic regression analysis was applied to evaluate baseline patients’ characteristics with the development of the primary adverse endpoint. A χ2 test for linear-by-linear associations was applied to evaluate trends in categorical outcomes, i.e., NYHA, through the follow-up period. Continuous variables are presented as mean ± SD and were compared between groups using Student’s t-test. All p-values are based on two-sided hypotheses.
Results
Baseline characteristics
The baseline characteristics of the 207 patients, 94 men (80 (7) years old) and 113 women (81 (6) years old), are presented in Table I; the youngest patient was 59 years old and the oldest was 98 years. All patients presented with chronic heart failure, the vast majority had hypertension, almost half had dyslipidaemia and almost one-third had type 2 diabetes mellitus. Most of the patients were overweight/obese and 1 out of 5 patients reported current smoking. No substantial differences were observed regarding the main baseline patients’ characteristics between the involved centres (all p-values > 0.23).
Table I
Baseline demographic and clinical characteristics of the 207 patients with severe aortic valve stenosis who underwent TAVI
Procedural characteristics
Concerning procedural characteristics, 99% of patients underwent TAVI through a transfemoral approach. Mean (SD) and median (quartiles) duration of hospitalization after the TAVI was 5 (3.4) days and 4 days (3.6), respectively. The procedure was performed using all the available sizes of the Myval THV; 151 (73%) patients with traditional sizes (i.e., 20, 23, 26 and 29 mm), 54 (23%) patients with the intermediate size (i.e., 21.5, 24.5 and 27.5 mm) THV, and 8 (4%) patients were treated using the extra-large sizes (i.e., 30.5 and 32 mm). The 21.5 mm Myval was implanted only in 8 (4%) patients, while most of the patients were treated using the 23.0, 24.5 and the 26.0 mm Myval THV (23%, 12%, 36%, respectively). In 91 (44%) patients, balloon pre-dilatation was performed, and in 13 (6.2%) patients post-dilatation was applied for better implant optimization.
Primary endpoint measures
All-cause mortality during the first 30 days following TAVI was observed in 16 (7.7%) patients (Table II) and was related mainly to periprocedural death rates. All deaths were in the elderly group and they were not related to the valve used. The likelihood of the primary endpoint during the first 30 days following TAVI was not associated with patients’ medical history, BMI or BSA, smoking habits, or any of the preoperative echocardiographic measurements (all p > 0.10). The primary endpoint was also not associated with STS score or EuroSCORE II surgical risk tools (all p > 0.10), but an inverse relationship was found between all-cause mortality and the Katz fragility index (p = 0.001). Among patients who incurred the primary adverse endpoint during the first 30 days following TAVI, the mean value of the Katz fragility index was significantly lower than in patients without primary adverse events (5.06 ±1.4 vs. 5.74 ±0.7, p = 0.01).
Table II
Primary end point(s) at 30-day, 1-year and two-year follow-up, timing of mortality and composite endpoints of the 207 patients with severe aortic valve stenosis who underwent TAVI with Myval
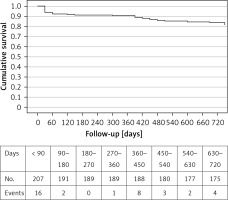
In Table II primary endpoint measures of the study are presented for the 30-day, 1-year and 2-year follow-ups. All-cause and cardiovascular (CVD) mortality rates were 9.7% and 4.3% at 1 year, and 17.4% and 9.7% at 2 years of follow-up, respectively. The Kaplan-Meier survival curve for all-cause mortality is illustrated in Figure 1. Seven (3.5%) patients were hospitalised for various reasons during the first 30 days after implantation, and in total, 19 (9.6%) patients were hospitalised for cardiovascular reasons during the follow-up period. The likelihood of the primary endpoint during 1 year and 2 years following TAVI was not associated with patients’ medical history, BMI or BSA, smoking habits, or STS score and EuroSCORE II surgical risk tools (all p > 0.10), but an inverse relationship again was observed with the Katz fragility index (p = 0.001). Patients who incurred the primary adverse endpoint during 1 year and 2 years after TAVI had lower values of the Katz fragility index than patients without primary adverse events (5.20 ±1.32 vs. 5.74 ±0.7, p = 0.01 and 5.08 ±1.36 vs. 5.81 ±0.57 p < 0.001, respectively).
Figure 1
Kaplan-Meier two-year survival curve for all-cause mortality of 207 patients with severe aortic valve stenosis who underwent a TAVI with a new generation, balloon expandable THV, Myval
Timing of mortality is also summarized in Table II. Periprocedural mortality was observed in 16 (7.7%) of the patients; 7 (3.4%) of them were cardiac and 5 of these cardiac events occurred ≤ 30 days after the index procedure and 2 of them occurred > 30 days but during the index hospitalization. Early mortality occurred in 4 patients; 2 of them were due to acute heart failure and 2 were due to pneumonia and sepsis. Late mortality occurred in 16 (7.7) patients and main causes were COVID-19, acute renal failure, heart failure, pneumonia and sepsis.
Composite endpoint measures
Composite endpoints of the study are summarized in both the graphical abstract and Table II. According to VARC-3 criteria, technical success was observed in 204 (99%) of the patients. Technical failure occurred in 3 (1.4%) patients due to major vascular complications. Device success was observed in 189 (91%) of the patients. There were 18 (9%) patients in whom device success was not achieved and most of them were due to death from any cause during the first 30-day follow-up period. Three of them were also those patients who developed technical failure due to major vascular complications. Clinical efficacy was not observed in 44 (21%) patients at the end of the 1-year follow-up. Among these 44 patients, 20 (10%) of them were patients who suffered from all-cause mortality. Clinical efficacy was observed in 163 (79%) of the patients, and early safety was observed in 161 (78%) of the patients. Early safety was not observed in 46 (22%) patients during the first 30-day follow-up period. Among these 46 patients, 23 (11%) required permanent pacemaker implantation, 16 (8%) died due to any reason and 7 other (3%) patients developed moderate to severe PVL.
Secondary outcomes at follow-up periods of 30 days and 2 years after the procedure
In Table III secondary endpoints of the study are presented for the 30-day and 2-year follow-ups. Permanent pacemaker implantation (PPI) was required in 11% of the patients within 30 days after the procedure, and in total, 12% required a PPI during the 2-year follow-up. Incidence of ≥ moderate PVL at 30 days, 1 year and 2 years of follow-up were 3.4%, 4.3% and 4.8%, respectively. The occurrence of ≥ moderate paravalvular leak at 30 days and 2 years is presented in Table III. Vascular complications occurred in 21 (7.7%) patients, major vascular complications were observed in 3 (1.4%) and minor vascular complications occurred in 18 (8.7%) patients. A post-operation peripheral balloon and peripheral covered stent were used in 11 (5.3%) patients. Prevalence of TIA and stroke was low (Table III); moreover, 2 (1.0%) patients had a disabling stroke event during the first 30 days following the procedure, and none had a non-disabling stroke. At 2-year follow-up, 4 (1.9%) patients had a disabling stroke and 1 (0.5%) had a non-disabling stroke.
Table III
Secondary end point(s) at 30-day and two-year follow-up of the 207 patients with severe aortic valve stenosis who underwent TAVI with Myval
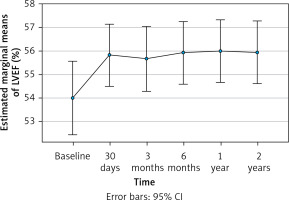
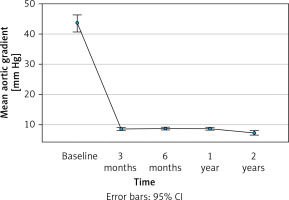
In Figure 2, the progression of LVEF (%) from baseline measurements through 2 years of follow-up of the patients is illustrated. Data analysis revealed a significant increase of LVEF from pre-TAVI (i.e., 53.9% [10.3%]) to the end of the first 30 days after TAVI (i.e., 55.8% (8.8%)) (p = 0.014), which was stabilised at around 56% through the end of the follow-up period. In line with the previous observations, the NYHA classification of the patients was significantly improved during the 2-year period (p < 0.001); as can be seen in Table III, 98% of the patients achieved NYHA class I or II by the end of 1-year of follow-up, whereas at the end of follow-up 43% were classified as NYHA I. The measurements of the mean pressure gradient across the stenotic aortic valve, an indicator of the severity of aortic stenosis, showed a progressive decline from pre-TAVI (43.5 [19.0] mm Hg) to 3-month post-TAVI (8.5 [2.9] mm Hg), and had stabilised by the end of the 2-year follow-up (7.3 [4.7] mm Hg) (p < 0.001) (Figure 3).
Discussion
The aim of this study was to evaluate, in a real-world setting, the performance and 2-year clinical outcome of a new generation BE THV, the Myval, among patients with degenerative SAS by using VARC-3 criteria.
In the present study, all-cause and cardiovascular mortality, as well as stroke and TIA rates, were in line with the other real world setting studies, during the entire follow-up period. [5, 11–16]. Beside the primary endpoint, The Myval THV showed very high technical (99%) and device success (91%), with very good clinical efficacy (79%), and early safety (78%). These composite VARC-3 defined endpoints were carefully evaluated in this real world study and clinical efficacy was assessed for the first time 1 year after the procedure.
García-Gómez et al. evaluated 100 patients (mean age was 80 ±6.5 years and mean STS was 2.4 ±0.8%) who underwent TAVI using the Myval in a retrospective study in nine European centres between September 2019 and February 2021 [16]. The femoral access route was used in 98% and intermediate sizes of the Myval were used in 39% of the patients. Procedural success was 99%. There were no cases of valve embolisation, annulus rupture, coronary occlusion, or procedural death. The PPI rate was 8%. Echocardiographic and functional improvement was maintained at 30 days and there were no deaths. There was moderate aortic regurgitation in 4% of patients. However, this study was done in low-risk patients, VARC3 criteria were not used and the follow-up period was restricted to only 30 days. A recent study, the SAPPHIRE prospective registry, by Testa et al. [5], that evaluated 100 consecutive patients from two Italian centres, who underwent TAVI for SAS, reported a successful implantation of the Myval, with no in-hospital mortality and 99% device success. Overall and CVD mortality rates observed in the SAPPHIRE registry were also similar to our study, both at 1-year and at 2-year follow-up. However, our study adds to the previous ones by evaluating a much larger cohort of patients who underwent TAVI using the Myval, for a longer-term period; especially compared to the SAPPHIRE registry results, the mean aortic gradients observed in this extended cohort are lower, and the clinical efficacy and technical success rates are higher.
In the present study, the primary endpoint was not associated with STS score and EuroSCORE II surgical risk tools, but an inverse relation was observed with the Katz fragility index (p = 0.001). Current data regarding the predictive ability and usefulness of STS and EuroSCORE II risk scores for TAVI are controversial, especially for different access sites. Discrepancies can also occur between these two scoring systems since some variables are not similar. For example, in a large US multicentre database, the STS-PROM performs better than EuroSCORE II for CABG. However, EuroSCORE II is a reasonable alternative in low-risk CABG patients and in those undergoing other cardiac surgical procedures. Physicians and clinical trials that use these scores recruit and treat patients who are at a lower risk than anticipated. Thus, decision-making should not solely be based on risk scores, but should comprise multidisciplinary heart team discussions and especially patients’ fragility status [17]. Patients in the present study were in the intermediate risk group and mortality rates were similar compared to other real world TAVI studies which were conducted in intermediate risk patients [18–20]. Frailty status is associated with higher mortality in this TAVI cohort and incrementally improves the well-validated STS or EuroSCORE II risk prediction models. Thus, our study also confirms other studies indicating that frailty assessment should continue to be part of the preprocedural assessment to further improve patient outcomes after TAVI [21, 22].
Besides single-arm registries with long-term follow-up, also reassuring data come from several clinical comparisons of Myval with other well-known and studied THV platforms. Delgado-Arana et al. compared a matched population of 103 patients who underwent TAVI with the Myval to 103 patients treated with the Sapien 3 [23]. While the early safety and clinical efficacy were comparable at 30 days, there was a lower need for PPI (5.8% vs. 15.5%, p = 0.02) and significantly lower mean gradients and PVL in the Myval group compared to the Sapien 3 group. The investigators attribute this to the use of intermediate sizes of the Myval in nearly 45% of the patients [16, 17]. Santos-Martinez et al. reported the results of conduction disturbances in a European registry of 1,131 patients who underwent TAVI with any of the following six THVs – Myval, Sapien 3, Evolut R, Accurate, Portico and Allegra [17]. Patients treated with Myval had the lowest rate of PPI (7.4%). In another single-centre retrospective cohort study, Barki et al. compared the results of 166 consecutive patients undergoing TAVI with either the Myval (n = 58) or the Evolut R THV (n = 108). [24]. Early device success was found to be significantly higher in the Myval group compared to the Evolut R group (94.8% vs. 83.3%, p = 0.048). At 30 days and at 6 months, the Myval group had a significant lower incidence of moderate PVL (6.9% vs. 19.8%, p = 0.039) and PPI (11% vs. 27.5%, p = 0.02). The incidence of all-cause mortality and disabling stroke was similar in both groups. Compared to these studies, roughly 1 out of 10 patients required PPI during the 2-year follow-up in our study. Although the PPI rates in the present study seem slightly higher than some other Myval studies, the results were similar to those of the study of Barki et al., and our PPI rates were still lower than in other studies which used conventional BE and self-expandable technologies. In a recent study, Sammour et al. sought to evaluate whether higher implantation of Sapien 3 reduces conduction abnormalities including the need for PPI [25]. Among 1028 patients, high deployment technique (HDT) was performed in 406 patients. The investigators found that thirty-day PPI rates were lower with HDT (5.5% vs. 13.1%; p < 0.001), as were rates of complete heart block (3.5% vs. 11.2%; p < 0.001) and new-onset left bundle branch block (5.3% vs. 12.2%; p < 0.001) [18]. In our study, most of the patients were treated by using classical BE THV implantation technique and our PPI rates still seem to be lower than for the BE S3 THV (11% vs. 13%). Thus, HDT with the Myval may also result in lower rates of PPI, and future studies are needed to evaluate this issue.
We believe that the present study confirms the available literature supporting the very high level of safety reached by TAVI with the Myval procedure. The performance of this novel BE THV has also been assessed in specific sub-sets of patients such as low-risk (for surgery), bicuspid aortic valve stenosis and valve-in-valve procedures (although it is not yet CE marked for this indication) and in dysfunctional stenosed right ventricular tract conduits with good clinical outcomes [26–28].
Among new generation TAVI devices, the Myval may have several advantages. First, the availability of intermediate and extra-large sizes may allow the operator to fit the prosthesis to the patient without concern for over/under expansion. Second, the valve is directly mounted on a balloon like a stent and can be inserted into the introducer sheath. This “deliver and implant” feature makes the procedure more intuitive, simpler and safer. Third, the valve can be retrieved through the introducer sheath and can be reinserted and reimplanted. If it has not been possible to cross the calcified aortic valve, the sheath design permits complete retrieval of the valve from the patient and reinsertion of the same valve. However, direct comparisons with other new platforms are lacking and are deemed necessary to reveal benefits and differences between these TAVI devices. The ongoing LANDMARK randomized controlled clinical trial (by Meril Life Sciences Pvt. Ltd.) comparing the Myval to the Sapien 3 and Evolut THVs and the COMPARE TAVI randomized controlled clinical trial comparing the Myval to the Sapien 3 are expected to provide important understanding of the clinical efficacy of this new THV [29, 30].
Strengths and limitations
Compared to other relevant studies, the superiority of this registry-based cohort study is due to the multi-centre design that simulates a real-world setting, the endpoints that are clearly defined according to the most recent VARC-3 criteria, and the relatively long-term follow-up, i.e., 2 years of more than 200 patients with severe aortic valve stenosis.
However, observational and registry-based studies, such as this one, cannot provide evidence for causality and are prone to selection bias. We tried to avoid the latter by selecting consecutive patients with aortic stenosis even if the decision to implant the Myval (instead of a different THV) was based on the operator’s discretion. The a-priori calculated statistical power for achieving the primary endpoint was 75%, which is considered a moderate level of evidence to support a hypothesis and correctly reject the null hypothesis. Randomised clinical trials and observational, registry-based studies with much longer follow-up are needed to further elucidate the performance of this novel Myval THV in a real-world setting.
In conclusion, in this cohort of patients with severe, native, aortic valve stenosis, the BE Myval was found to be safe and effective in up to 2 years’ follow-up. The present study expanded the findings from previous studies on the Myval THV technology. Randomized data from the LANDMARK and COMPARE TAVI trials are awaited to confirm these promising clinical data.