Introduction
In recent years, membranous nephropathy (MN) has increased in prevalence, and is a common glomerulonephritis [1, 2]. Studies have also shown that membranous nephropathy is the most common glomerulonephritis in the elderly [3–6]. Membranous nephropathy is considered as an organ-specific autoimmune disease, and is histologically defined by subepithelial immune deposits. The major membranous nephropathy target antigen is podocyte phospholipase A2 receptor (PLA2R) [7]. It has been reported that approximately 70% and 80% of total idiopathic membranous nephropathy patients are found with elevated serum anti-PLA2R antibodies levels and enhanced glomerular PLA2R deposits, respectively [8].
B lymphocytes activated by T cells produce antibodies, which play an essential role in glomerular immunoglobulin deposits and complement activation [9, 10], and B cells have been suggested as antigen-presenting cells involved in the pathogenesis of MN [11]. CD20 is a pan-B cells marker due to its expression in the pre-B stage of B cells and absence after differentiating to plasma cells [11]. CD19+/CD20+ plasma cells may contribute to autoantibody and alloantibody production [12, 13].
Rituximab is a monoclonal antibody that specifically binds to the CD20 antigen. In recurrent MN [14–16], anti-CD20 antibody with classical immunosuppressant treatment may block the proliferation of B cells and the production of pathogenic antibodies in patients. Rituximab is also effective in treating other conditions associated with autoantibody production, such as systemic lupus erythematosus [17] and rheumatoid arthritis [18]. Although the effects of rituximab on MN have not been characterized in animal studies, clinical trials and case reports have reported that rituximab is associated with good remission rates and is safe in patients with MN. So far, only Zou et al. [19] have conducted studies prior to 2016 with 12-month follow-up to analyze the efficacy and safety of rituximab in idiopathic membranous nephropathy. However, Cravedi [20] suggests that nephrologists should note the limitations of this study and warn against an oversimplified interpretation of the data. Based on this literature, we analyzed the role of rituximab in membranous nephropathy in different stages of membranous nephropathy, different follow-up periods, and administered doses and adverse effects. This meta-analysis will provide evidence for the application of rituximab in MN.
Material and methods
Search strategy
The keywords “rituximab”, “CD20 antibody”, “glomerulonephritis, membranous”, “membranous nephropathy”, “idiopathic membranous nephropathy” and “membranous glomerulonephritis”. “(rituximab OR CD20 antibody) AND (glomerulonephritis membranous OR membranous nephropathy OR idiopathic membranous nephropathy OR membranous glomerulonephritis)” was entered into PubMed, Embase, Cochrane Library and ClinicalTrials.gov for search without language or publication date limitations.
Inclusion and exclusion criteria
We included and excluded relevant studies according to the following criteria. Primary membranous nephropathy was eligible, and secondary membranous nephropathy was excluded. Rituximab as the first line in membranous nephropathy was included, and combined therapy or second line therapy was excluded. Patients were over 18 years old; pediatric trials were not considered. Reviews, case reports and editorials were excluded, and only randomized controlled trials, prospective studies, and retrospective studies were included in this meta-analysis.
Outcome measures and data collection
The numbers of complete remissions (CR) and partial remissions (PR) were extracted to measure the efficacy of rituximab in membranous nephropathy at 12 months and at 24 months. Basic information was collected from each study, including author, publish year, rituximab dose, study design, total subjects in completed trials and period of follow-up. Baseline values of proteinuria, serum albumin, serum creatinine and estimated glomerular rate (eGFR) of patients were also recorded. Finally, adverse effects were included. The most common adverse effects of rituximab in MN are infusion reactions, infections, cardiovascular events, bullous dermatitis and small patches of hair loss and thinning, and tumors. Infusion reactions included symptoms of skin rash, flu-like symptoms, and a metallic taste.
Statistical analysis
The primary aim of treatment is to reduce chronic proteinuria. The 2012 KIDGO guideline of idiopathic membranous nephropathy proposes a relationship between 24 h proteinuria and disease progression. We divided patients into low-risk (proteinuria was < 4 g/day), medium-risk (proteinuria was 4–8 g/day) and high-risk groups (proteinuria was > 8 g/day) according to their baseline level of proteinuria. The role of rituximab in membranous nephropathy was analyzed at different stages of disease progression and follow-up periods (12 months and 24 months). CR and PR values were extracted from the included studies, and pooled using the method of the inverse of the variance with logit transformed proportions [21]. Review Manager Version 5.3 software was used for further analyses. The results were converted according to the formulae below [22], and the values were considered to denote the efficacy of rituximab. Dichotomous data were expressed by the odds ratio (OR), and the 95% confidence intervals (95% CI) were used for the recruited studies. A p-value less than 0.05 was considered as statistically significant. The heterogeneity was assessed withIsquare (I2). When I2 was less than 50% [23], a fixed-effects model was used; otherwise, a random-effects model was used [24, 25].
Results
Search results
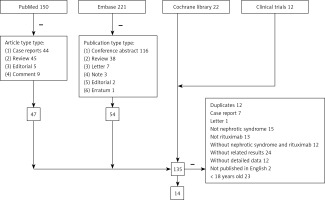
Results of every database are displayed in Figure 1. There were 150, 221, 22 and 12 articles in PubMed, Embase, Cochrane library and ClinicalTrials.gov, respectively. Removing article types based on the inclusion criteria and duplicates, 14 articles including 17 studies were included in this meta-analysis.
Study characteristics
Table I displays the basic characteristics of the included reports. There were 2 retrospective reports [26, 27] and 12 prospective reports [28–39]. There are many administration protocols for rituximab, including four weekly doses of 375 mg/m2 (the most common), 15 days part of 1 g and titrated to circulating B cells. The total number of completed studies was 364. The follow-up period varied; eight studies [27, 30, 32, 33, 35, 37, 39] contained data to 12 months, four studies [28, 29, 36, 38] included data to 24 months. Patient characteristics of 17 studies are shown in Table II. Based on baseline proteinuria, no studies were classified as low-risk, three studies [26, 27, 31] were included in the medium-risk group, and twelve studies [28–30, 32–39] were classified as high-risk. Seven studies [27–29, 32, 34–36] reported that rituximab was administered in four weekly rituximab doses of 375 mg/m2. Two studies [30, 31] reported that rituximab (375 mg/m2) was administered once or twice. Two reports [37, 38] contained a 1 g dose of rituximab twice, but the number of PR and CR could not be extracted.
Table I
Studies included in this meta-analysis
Table II
Baseline characteristics of included studies
| Author, year | PMID | Age [years] | Gender | Proteinuria [g/day] | Serum albumin | Serum creatinine [mg/dl] | eGFR [ml/min/1.73 m2] |
|---|---|---|---|---|---|---|---|
| Cravedi, 2011 | 21508634 | 50.1 ±12.3 | 1 female, 10 male | 10.3 (5.8–13.8) | 2.1 ±0.6 g/dl | 1.1 ±0.4 | > 20 |
| Irazabal, 2013 | 22987142 | 49.0 ±13.0 | 3 female, 17 male | 11.9 ±4.9 | 2.7 ±0.6 g/dl | 1.5 ±0.5 | 72.4 ±33.2 |
| Moroni, 2017 | 27387472 | 52.8 ±15.2 | 11 female, 23 male | 11.9 ±8.2 | 2.4 ±0.6 g/dl | 1.3 ±0.6 | 67.2 ±30.8 |
| Bagchi, 2018 | 29942496 | 33.3 ±12.3 | 33.3% were females | 6.2 ±2.2 | 2.5 ±0.5 g/dl | 0.9 ±0.3 | 95.8 ±26.9 |
| Souqiyyeh, 2015 | 25579715 | 37.4 ±9.5 | Total 25 | 6.2 ±4.7 | 34.1 ±6.2 g/dl | – | 96.1 ±46.1 |
| Ruggenenti, 2006 | 17699281 | 51.2 ±13.2 | 5 female, 4 male | 8.9 ±5.3 | 2.2 ±0.6 mg/dl | 1.0 ±0.3 | 95.6 ±20.3 |
| Wang, 2018 | 29149305 | 47.3 ±17.6 | 6 female, 30 male | 12.3 ±5.9 | 21.9 ±5.8 g/l | 2.1 ±1.4 | 55.7 ±33.9 |
| Aleš Rigler, 2017 | 28664837 | 51.2 ±11.8 | 7 female, 19 male | 7.9 ±4.4 | – | 1.5 ±0.7 | 80.4 ±43.8 |
| Ruggenenti, 2012 | 22822077 | 51.5 ±5.9 | 28 female, 72 male | 9.1 (5.8–12.8) | 2.2 ±0.6 g/dl | 1.2 (0.97–1.7) | 60.0 ±4.0 |
| Ruggenenti, 2003 | 12819245 | 52.8 ±19.6 | 5 female, 3 male | 8.6 ±3.9 | 2.7 ±0.5 mg/dl | 1.5 ±0.8 | – |
| Fervenza, 2010 | 20705965 | 48.6 ±12.9 | 17 male 3 female | 11.9 ±4.9 | 2.7 ±0.6 g/100 ml | 1.5 ±0.5 | 72.4 ±33.2 |
| Fervenza, 2008 | 17943078 | 47.0 ±8.0 | 5 female, 3 male | 13.0 ±5.7 | 2.3 ±0.6 g/100 ml | 1.4 ±0.5 | 85.2 ±13.6 |
| Beck, 2011 | 21784898 | 48.2 ±10.9 | 5 female, 28 male | 12.4 ±5.1 | – | 1.5 ±0.5 | 77.9 ±31.3 |
| Cravedi, 2007 | 17702725 | 57.0 ±13.0 | 67% male | 10.3 ±8.9 | 2.3 ±0.8 g/dl | 1.4 ±0.5 | > 20 |
| Cravedi*, 2007 | 17702725 | 55.0 ±15.0 | 67% male | 9.1 ±3.8 | 2.4 ±0.6 g/dl | 1.5 ±0.7 | > 20 |
Efficacy of rituximab in membranous nephropathy
Fifteen studies [26–39] were included to assess the efficacy of rituximab in MN. The I2 = 37%, fixed model was applied, which suggested that the heterogeneity between included documents was minimal. The pooled OR of initial results was 1.38 (95% CI: 1.12–1.71; p = 0.003; Figure 2 A), and after conversion, the pooled OR of overall PR and CR remission rate was 0.58 (95% CI: 0.53–0.63; p = 0.003), which was statistically significant.
Figure 2
The efficacy of rituximab in membranous nephropathy (MN). A – The overall remission rate of rituximab in MN. B – The remission rate of rituximab in medium-risk group. C – The remission rate of rituximab in high-risk group
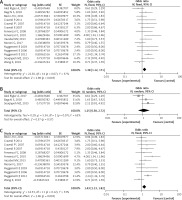
The baseline proteinuria of three studies [26, 27, 31] met criteria of the medium-risk group. The I2 = 63%, a random model, indicated heterogeneity of 1.25 (95% CI: 0.58–2.72; p = 0.57; Figure 2 B) between the included studies. After conversion, the pooled OR of overall PR and CR remission rate in the medium-risk group was 0.56 (95% CI: 0.36–0.73), not statistically significant.
According to baseline proteinuria, twelve studies [28–30, 32–39] were included in the high-risk group. The I2 = 33%, a fixed model was used, indicating a minimal heterogeneity between the included studies of 1.43 (95% CI: 1.13–1.82; p = 0.003; Figure 2 C). After conversion, the pooled OR of overall PR and CR remission rate in the high-risk group was 0.59 (95% CI: 0.53–0.65), which was statistically significant.
Eight studies [27, 30, 32, 33, 35, 37, 39] were assessed to analyze the efficacy of rituximab at the 12-month follow-up. The heterogeneity was low, with an I2 of 25%. The pooled OR of overall PR and CR was 1.06 (95% CI: 0.77–1.45; p = 0.72). After transformation, the pooled OR of overall PR and CR remission rate in 12-month follow-up was 0.51 (95% CI: 0.43–0.59), not statistically significant.
Four studies [28, 29, 36, 38] were assessed to analyze 24-month efficacy of rituximab. Since the I2 was 69%, a random model was executed, indicating heterogeneity between studies. The pooled OR of initial PR and CR rate was 2.40 (95% CI: 0.92–6.26; p = 0.07). After transformation, the pooled OR of overall PR and CR remission rate at the 24-month follow-up was 0.71 (95% CI: 0.48–0.86), not statistically significant.
The efficacy of rituximab administered at 375 mg/m2 × 4 was then assessed. The I2 was 44%; a fixed model indicated heterogeneity between the reports, with the pooled OR of initial PR and CR of 1.7 (95% CI: 1.24–2.35; p = 0.001). After conversion, the pooled OR of overall PR and CR was 0.63 (95% CI: 0.55–0.70), statistically significant. Studies of rituximab administered at 375 mg/m2 once or twice were not included.
Safety of rituximab in membranous nephropathy
The most common adverse effects of rituximab in MN are infusion reactions, infections, cardiovascular events, bullous dermatitis and small patches of hair loss and thinning, and tumors. Infusion reactions included symptoms of skin rash, flu-like symptoms, and a metallic taste. The majority of these were acute and disappeared after treatment infusions were stopped or methylprednisolone infusion. Some resolved without any treatment. Cardiovascular-related events were atrial fibrillation, myocardial infarction and hypotension. These symptoms typically appeared several months following rituximab infusion. No treatments were prescribed in the included studies. Infectious included soft tissue infections of lower extremities, herpes zoster, respiratory tract infection and pneumonia. These symptoms developed over 1–5 months after rituximab infusion. Oral antibiotics or anti-viral drugs were successfully used to treat these conditions. Bullous dermatitis often appeared 1 day after RTX. Hair loss and tumors were rare.
Six studies [28, 34–37, 39] were included to analyze the infusion reaction rate of rituximab in MN. The I2 was 74%, so a random model was applied. Heterogeneity was present in the included studies. The pooled OR of initial result was 0.33 (95% CI: 0.14–0.80; p = 0.01; Figure 3 A). After transformation, the pooled OR of infusion reaction rate of rituximab was 0.25 (95% CI: 0.13–0.44), which was statistically significant.
Figure 3
The safety of rituximab in membranous nephropathy (MN). A – The infusion reaction rate of rituximab in MN. B – The cardiovascular related event rate of rituximab in MN. C – The infection rate of rituximab in MN
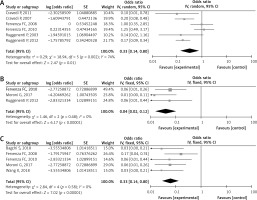
Three studies [30, 34, 36] were assessed to analyze the cardiovascular-related event rate of rituximab in MN. Due to an I2 of 0, there was no heterogeneity between the studies. The pooled OR of initial result was 0.04 (95% CI: 0.02–0.12; p < 0.00001; Figure 3 B). After conversion, the pooled OR of rate was 0.04 (95% CI: 0.02–0.11), which was statistically significant.
Five studies [30, 31, 33, 36, 37] reported infection with rituximab treatment of MN. The pooled I2 = 0%, a fixed model was conducted, with no heterogeneity between the reports. The pooled OR of initial result was 0.06 (95% CI: 0.03–0.14; p < 0.00001; Figure 3 C), and after transformation, the pooled OR of rate was 0.06 (95% CI: 0.03–0.12), statistically significant.
Discussion
Proteinuria is a marker of membranous nephropathy. With 4–120 months of observation, approximately 30–40% of patients can achieve spontaneous complete remission [40, 41]. With optimal supportive care combined with classical immunosuppressive therapy, less than 30% of cases progress to severe renal insufficiency [42–44]. Clinical studies indicate that severe proteinuria is a risk factor for progression of idiopathic membranous nephropathy to end-stage renal disease [45]. Thus, urine protein data are valuable to inform treatment-related decisions [46, 47]. Proteinuria is also an indirect indicator of autoimmune activity and disease severity [48]. In this meta-analysis, the included studies were divided into high-risk, medium-risk and low-risk groups according to baseline proteinuria levels. The overall remission rate was 58% (p = 0.003), and the remission rate of the medium-risk and high-risk groups were 56% (p = 0.57) and 59% (p = 0.003), respectively, lower than some prospective studies included [26, 28, 31, 32, 34, 39]. The effective ratio of rituximab in some reports approached 80% [29, 35, 36]. In the randomized controlled trial from Dahan et al. [49], the rate of CR or PR was 35% in the primary 6-month endpoint, but after an extended median follow-up of 17 months, 64.9% of rituximab-treated patients were in remission. In the prospective study from Aleš Rigler et al. [27], the remission rate was 37.9% following rituximab treatment. They attributed the results to a short follow-up period and low dose of rituximab. Based on this, we conducted another subgroup analysis. The remission rates were 51% (p = 0.72) and 71% (p = 0.07) in 12-month follow-up and 24-month follow-up, respectively. To some extent, the remission rate of rituximab was related to the observation time. Considering the dose administered, the pooled remission rate with rituximab (375 mg/m2 × 4) was 63%, similar to previous reports.
Rituximab is well tolerated in most patients [33]. In our meta-analysis, the most common adverse effects were infusion reaction (25%, p = 0.01); others were infection (6%, p < 0.00001) and cardiovascular events (4%, p < 0.000001). It has also been safer over the past 10 years compared to other immunosuppressive agent treatments for this disease [50, 51]. Rituximab ameliorated proteinuria and had minimal adverse effects on quality of life [52], where patients receiving cyclophosphamide had higher rates of hospitalization, liver toxicity, infection, and cardiovascular and thrombotic events [53]. In addition, rituximab can act in a B-cell-independent manner to prevent the disruption of the actin cytoskeleton and podocyte apoptosis [54].
Overall, rituximab is safe and effective alternative treatment. We speculate that the underlying mechanism is that rituximab is a monoclonal antibody that specifically binds to CD20 antigen, blocking the proliferation of B cells and the production of pathogenic antibodies in MN patients. The role of B cells in MN is unknown. B cells play an important role in the immune pathogenesis of glomerulonephritis, but their biological actions still need to be understood in order to determine the time, duration and conditions of the optimal therapeutic response to B cell targeting methods [55]. Furthermore, severe MN may involve spontaneous remission without treatment, and its progression was difficult to predict, which explains the treatment uncertainty that still exists today [44, 56, 57]. Rituximab treatment for the depletion of B cells to reduce urinary protein and promote clearance of autoantibodies [58–61] is still debated based on recent trials and metanalyses. A lack of response to rituximab treatment may be due to a lack of CD20 molecules in the plasma cell produced antibodies [62]. Therefore, B cell depletion may not be sufficient to maintain sustained remission [27, 30]. Multiple doses and protocols of rituximab have been used in different studies [20]. Some researchers have observed that the half-life of rituximab in MN patients is shorter than with other diseases, and attribute this to the molecular weight of rituximab (145 kDa), which may not be cleared in the urine [63, 64]. Some patients with rheumatoid arthritis [65] and idiopathic membranous nephropathy [49] are resistant to CD20 monoclonal anti-B cells, and this may increase the instability of rituximab in patients with MN.
There were some limitations in the present meta-analysis. Not all included studies were randomized controlled trials, making it more difficult to assess overall efficacy. Second, the number of patients included in our meta-analysis was small. The ideal method to classify membranous nephropathy is based on pathological manifestation, not proteinuria. However, few studies included pathological results, and classifying the severity of MN based on proteinuria is appropriate for clinical applications.
In conclusion, rituximab is a promising, safe and effective alternative treatment for MN. Additional randomized controlled trials of rituximab for MN are needed as well as additional studies on the role of B cells in MN.