Introduction
Atrial fibrillation (AF) and atrial flutter (AFl) are the most common types of atrial arrhythmia (AA) and are both important risk factors for ischaemic stroke (IS). Compared with patients with non-cardioembolic strokes, AF-related strokes constitute the most severe IS subtype, resulting in greater disabilities, higher mortality rates, and higher treatment costs [1]. Although AF is regarded as a high-risk cardiac source of cerebral embolism, approximately 20% of patients with AF have multiple potential stroke aetiologies [2]. This finding is highly important because these patients remain at a substantial risk for IS even when appropriately treated with oral anticoagulants. Although many trials have evaluated the mechanisms of IS in AF patients and the impact of AF on stroke severity, data concerning the prognosis and aetiology of AFl-related strokes are lacking.
Our aim was to compare the clinical course of cerebrovascular accidents (CVA), namely IS or transient ischaemic attacks (TIAs), between patients with sustained AFl or permanent AF (PmAF).
Material and methods
This study involved a retrospective analysis of patients consecutively admitted to a tertiary care centre over a period of 3 years (1.01.2013–31.12.2015) due to IS or TIA with known or newly detected AA, namely AFl or AF. The AF classification and management recommended by the European Society of Cardiology was used (2016) [3]. The spontaneous conversion of AF to a sinus rhythm or termination within 48 h of onset was considered paroxysmal (PAF). If cardioversion was required or AF persisted longer than 7 days, it was classified as persistent. Atrial fibrillation refractory to cardioversion and long-lasting AF was classified as PmAF. We included patients with sustained AFl or PmAF and excluded patients with paroxysmal AFl (n = 3) or PAF (n = 170) from the analysis due to the low number of patients with paroxysmal AFl and because CVA in patients with PAF and PmAF may have different courses with less severe strokes in PAF [4, 5]. While the reason is unclear, compared to patients with chronic AF, the relatively short episodes of AF in patients with PAF probably lead to the formation of thrombi of a relatively small size, which embolise more distally and cause infarcts of smaller volume. The AF group (AF, n = 490) comprised patients with persistent or long-standing persistent AF (n = 65, 13.3%), 3 patients with PmAF, who also had short episodes of AFl based on Holter ECG, and patients with PmAF (n = 422, 86.7%). Due to the low number of patients with AFl and haemorrhagic stroke (n = 2), we did not include patients with this type of stroke in the study.
According to national and international guidelines, all patients received standardised stroke treatment and diagnosis, consisting of clinical examinations, cerebral imaging (CT scans at admission and discharge), and carotid/vertebral and transcranial ultrasonography [6–8]. Electrocardiograms (ECGs) were performed in all patients upon admission, and 24-h Holter ECGs were performed in all patients with AFl and most patients with AF (n = 461, 94%), which were analysed by an experienced cardiologist blinded to the stroke status. All patients with first-detected AF or AFl underwent transthoracic echocardiography (TTE) to exclude valvular disease and establish the left ventricular ejection fraction (LVEF). All patients were haemodynamically stable during hospitalisation, and no patients required cardioversion. The severity of stroke was categorised at admission and discharge according to the level of disability on the modified Rankin functional scale (mRS; 0–6 scores, where 0 = no neurological deficit and 6 = death) and the National Institutes of Health Stroke Scale (NIHSS). The patients were classified as nondependent (mRS ≤ 2 points) or dependent (mRS ≥ 3 points) at admission. A poor stroke outcome was defined if the stroke caused dependence or death during hospitalisation. We defined cardiovascular death as death resulting from an acute myocardial infarction; sudden cardiac death; and death due to heart failure, stroke, cardiovascular procedures, cardiovascular haemorrhage, or other cardiovascular causes. The Causative Classification of Stroke (CCS) method was used to classify the stroke aetiology in accordance with the Stop Stroke Study Trial of Org 10172 in Acute Stroke Treatment (SSS-TOAST) criteria [9]. These criteria integrate the results of clinical evaluations, brain imaging, vascular and heart examinations, and work-up for uncommon causes of stroke. The strokes were categorised into one of the following categories: cardioembolism, large-artery atherosclerosis, small-vessel occlusion (lacunar stroke – LS), other determined aetiologies (e.g. vasculopathy, vasculitis, a hypercoagulable state, or haematological disorder), and cryptogenic disease (due to insufficient information or an undetermined cause). Probable small-artery occlusion was recognised in patients with imaging evidence of a single clinically relevant acute infarction < 20 mm at the largest diameter within the territory of the basal or brainstem-penetrating arteries in the absence of any other pathology in the parent artery at the site of the origin of the penetrating artery and presenting with a classical lacunar syndrome. If multiple potential causes existed, the patient was assigned to the undetermined cause group. The CCS has been shown to have good to excellent intrarater and interrater reliability [10].
Prestroke therapeutic anticoagulation was recognised in patients who were treated with vitamin K antagonists (VKA), had International Normalised Ratio (INR) values ≥ 2.0 at admission, or had well-documented regular treatment with novel oral anticoagulants (NOAC) before stroke onset. We collected data regarding the following vascular risk factors: hypertension, diabetes mellitus, dyslipidaemia, ischaemic heart disease, previous stroke/TIA, current smoking, moderate or heavy alcohol consumption (≥ 2 standard alcoholic drinks per day), and body mass index (BMI). Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, any use of antihypertensive drugs, or any self-reported history of hypertension. Diabetes mellitus was defined as a fasting glucose level ≥ 7.0 mmol/l, a non-fasting glucose concentration ≥ 11.1 mmol/l, any use of glucose-lowering drugs, or any self-reported history of diabetes. Dyslipidaemia was defined as a serum triglyceride level ≥ 1.7 mmol/l, low-density lipoprotein cholesterol ≥ 3.6 mmol/l, high-density lipoprotein cholesterol ≤ 1.0 mmol/l, any use of lipid-lowering drugs, or any self-reported history of dyslipidaemia. Ischaemic heart disease was defined as a history of angina or myocardial infarction.
Statistical analysis
The statistical analyses were performed with the Statistica 17.0 software (StatSoft Inc., USA). The quantitative and qualitative demographic characteristics were summarised, and the data were tabulated and tested for normality with the Shapiro-Wilk test. To compare the groups, Mann-Whitney U and Student’s t-tests were used to analyse the continuous/ordinal variables, and χ2, Fisher-Freeman-Halton, and Fisher's exact tests were used to analyse the categorical variables. The results are shown as the means ± SD or counts and percentages. Univariable and multivariable logistic stepwise regression analyses were used to evaluate the relationship between all studied parameters and a poor stroke outcome (mRS ≥ 3). Age, sex, baseline mRS score, type of IS, and prestroke therapeutic anticoagulation were selected on the basis of clinical plausibility and previous literature reviews and were included in the multivariable regression model to assess their influence. The statistical significance level was set at p-value < 0.05 for all analyses.
Results
Of the 2454 patients admitted due to IS or TIA, 701 (28.6%) patients had AA, including 41 (5.8%) patients with AFl (38 patients with permanent AFl and 3 patients with paroxysmal AFl), 490 patients with PmAF (69.9%), and 170 (24.2%) patients with PAF. In this cohort, 202 (28.8%) patients were newly diagnosed with AA (46 patients were diagnosed with PAF, 151 patients were diagnosed with persistent AF, and 5 patients were diagnosed with AFl). After the exclusion of patients with paroxysmal AF and AFl, the final group included in the analysis consisted of 528 patients as follows: 7.2% of the patients had AFl, and 92.8% of the patients had AF.
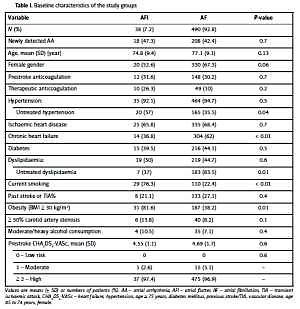
The mean age, sex distribution, frequency of prior cerebrovascular events, and prestroke CHA2DS2-VASc scores were similar between the AFl and AF patients; however, the prevalence of chronic heart failure in the AF patients was higher than that in the AFl patients, and the AFl patients were more frequently obese and active smokers (Table I). Only 49% of the patients with previously known AFl and AF received prestroke anticoagulation; most of these patients were treated with vitamin K anticoagulants (7/12 (58%) vs. 101/148 (68%), respectively, p = 0.2), while the other patients received NOACs. The patients with AFl, who received prestroke VKAs or NOACs, had therapeutic anticoagulation (10/12, 83%) more often than the patients with AF (49/148, 33.1%; p = 0.01). Among the patients with first-detected AA, the patients with AF had lower mean LVEFs (50.2 ±10.5% vs. 59.17 ±6%, p < 0.01) than the patients with AFl. Similar proportions of patients with AFl and AF received thrombolytic treatment for acute stroke (5.2% vs. 6.9%, p = 0.3). The patients with AFl had transient symptoms (TIA) more often, and those with IS were more often nondependent upon admission and discharge, had lower mean mRS and NIHSS scores, and had lower in-hospital mortality than the patients with AF (Table II). The causes of death in both groups were similar and related to the following cardiovascular reasons: consequences of index stroke (3 patients with AFl (100%) and 84 (80%) with AF, p = 0.7), recurrent stroke (9 patients (8.7%) with AF), and sudden cardiac death or pulmonary embolism (11 patients with AF (11.3%)). The stroke aetiologies differed between the studied groups. Most IS cases in the AF group were classified as cardioembolic strokes (74.9% vs. 39.5% in AFl); however, LS, which is caused by a small artery occlusion, was more common in the AFl group (47.4% vs. 14.3% in AF). The mean hospitalisation durations of the AFl (11.9 ±4 days) and AF (12.8 ±7 days) patients were similar (p = 0.4).
Table I
Baseline characteristics of the study groups
[i] Values are means (± SD) or numbers of patients (%). AA – atrial arrhythmia, AFl – atrial flutter, AF – atrial fibrillation, TIA – transient ischaemic attack, CHA2DS2-VASc – heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke/TIA, vascular disease, age 65 to 74 years, female.
Table II
Presumed mechanism and in-hospital course of stroke in patients with AFl and AF
In the univariable analysis, patients with AF and non-lacunar stroke with more severe neurological deficits at baseline, cardioembolic risk factors, and higher CHA2DS2-VASc scores were more likely to have unfavourable stroke outcomes. Atrial fibrillation, admission mRS, lack of prestrike anticoagulation, non-lacunar stroke, heart failure, and diabetes were independently associated with poor outcomes in the multivariable analysis, while patients who were active smokers had a lower risk (Table III).
Table III
Univariate and multivariate analysis of factors associated with poor stroke outcome (modified Rankin scale 3–6)
| Characteristics | Univariate OR (95% CI) | P-value | Multivariate OR (95% CI) | P-value |
|---|---|---|---|---|
| AA : AF vs. AFl | 9.14 (3.19–26.1) | < 0.001 | 8.6 (1.2–57) | 0.02 |
| Age* | 1.05 (1.03–1.08) | < 0.001 | 1.02 (0.98–1.06) | 0.22 |
| Female gender | 2.06 (1.4–2.98) | < 0.001 | 0.9 (0.4–1.9) | 0.8 |
| Admission mRS* | 10.8 (7.7–15.3) | < 0.001 | 16.6 (9.8–28) | < 0.001 |
| Stroke aetiology: | ||||
| Lacunar vs. non-lacunar stroke | 0.11 (0.05–0.21) | < 0.001 | 0.1 (0.03–0.31) | < 0.001 |
| No prestroke anticoagulation | 4.35 (1.97–9.6) | < 0.001 | 6.1 (1.1–33) | 0.03 |
| Hypertension | 0.88 (0.41–1.87) | 0.75 | – | |
| Ischemic heart disease | 3.5 (2.35–5.19) | < 0.001 | – | |
| Chronic heart failure | 4.28 (2.93–6.24) | < 0.001 | 14.2 (5.8–34) | < 0.001 |
| Diabetes | 1.92 (1.36–2.73) | < 0.001 | 2.9 (1.3–6.5) | < 0.01 |
| Dyslipidaemia | 0.55 (0.39–0.78) | < 0.01 | – | |
| Current smoking | 0.62 (0.33–0.81) | < 0.01 | 0.92 (0.39–0.99) | 0.04 |
| Past stroke/TIA | 1.19 (0.76–1.64) | 0.56 | – | |
| Obesity (BMI ≥ 30 kg/m2) | 0.69 (0.48–0.98) | 0.04 | – | |
| ≥ 50% carotid artery stenosis | 0.64 (0.35–1.19) | 0.16 | – | |
| Moderate/ heavy alcohol consumption | 1.9 (0.99–3.86) | 0.05 | – | |
| Prestroke CHA2DS2-VASc | 1.35 (1.2–1.5) | < 0.001 | – | |
| Old subcortical lacunes | 1.1 (0.7–1.5) | 0.58 | – | |
| Leukoaraiosis | 1.5 (1.07–2.36) | 0.02 | – | |
| Admission SBP* | 1.01 (1.003–1.01) | < 0.01 | – | |
| Admission DBP* | 1.01 (1.006–1.03) | < 0.01 | – | |
| Admission NIHSS* | 2.25 (1.89–2.67) | < 0.001 | – | |
AA – atrial arrhythmia, AFl – atrial flutter, TIA – transient ischaemic attack, DBS – diastolic blood pressure, SBP – systolic blood pressure, mRS – modified Rankin scale, NIHSS – National Institute of Stroke scale, CHA2DS2-VASc – heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke/TIA, vascular disease, age 65 to 74 years, female.
Discussion
Our data show that the in-hospital course of IS in patients with AFl was more favourable than that in patients with AF. The patients with AF had higher in-hospital mortality and were more often dependent at admission and discharge from the hospital than the patients with AFl. We also revealed that cardioembolic stroke, heart failure, diabetes, and lack of prestroke anticoagulation were associated with a poor outcome following stroke in our cohort. This study also confirmed that AFl is a rare arrhythmia, accounting for only 5.8% of patients hospitalised for IS or TIA within 3 years.
To the best of our knowledge, this study provided the first information regarding the course of CVA in patients with AFl. Atrial fibrillation is a well-established independent predictor of poor stroke outcomes, large territorial infarcts, and secondary haemorrhagic transformation; however, the explanation for the more favourable stroke outcomes among the AFl patients is unclear. This finding is possibly a result of the more frequent non-cardioembolic CVA, mainly LS, which is usually less severe than those of cardiac origin [11]. Although pre-stroke anticoagulation and mean CHA2DS2-VASC scores were similar between the groups, the patients with AFl were more often active smokers and obese and had untreated hypertension and hypercholesterolaemia, which are well-known risk factors for atherothrombotic lacunar strokes. The neuroimaging findings revealed that the AFl patients had more frequent acute subcortical strokes and less frequent cortical strokes, which are typical cardioembolic strokes, than the AF patients. The multivariable analysis demonstrated that AF, heart failure, diabetes, and lack of prestroke anticoagulation, all of which constitute well-known risk factors for cardioembolism, were independently related to unfavourable stroke outcomes. The finding of a lower frequency of cardioembolic strokes associated with AFl than with AF is consistent with the findings reported in a study examining a large Medicare database, which showed that the cardioembolic risk in patients with AFl (relative risk – RR = 1.41) was higher than that in patients without AA but lower than that in patients with AF (RR = 1.64) [12]. However, the clinical thromboembolic risk, which was assessed by the CHA2DS2-VASc in the current analysis, was similar between the studied groups, and cardioembolism as the cause of some LS in AFl or AF cannot be excluded [13]. This study also revealed that current smoking was an independent predictor of a favourable clinical outcome in ischaemic stroke. Similar observations have recently been reported in both cardioembolic and non-cardioembolic strokes, but the mechanism is unknown [14]. Smokers supposedly have a better cerebral collateral supply or may be better preconditioned for ischaemia due to the increased plasma levels of carbon monoxide and episodic hypoxia [15].
Hypertension plays a significant role as a cardiovascular risk factor and has been shown to promote both AF and AFl. Most patients with AFl and AF in our study had hypertension, but those with AFl had more frequent untreated hypertension or higher baseline DBP. Although these factors were unrelated to the stroke outcome in our study, evidence from multiple-cohort studies has confirmed the presence of a strong association between hypertension and AF, leading to the inclusion of blood pressure indices as clinical risk scores for AF prediction. Despite the availability of diagnostic methods and choice of different antihypertensive drugs, further improvement of awareness of hypertension control is highly relevant for many patients with AF or AFl [16]. Hypertension is also a strong predictor of arrhythmia progression from paroxysmal to chronic AF, i.e. according to the RECORD-AF study, arrhythmia progression was more common among patients with PAF, who developed sustained AF (OR = 1.5, 95% CI: 1.1–2.0) [17].
Our results are consistent with previous observations that AF carries an excess risk of poor outcomes [18]. This increased risk may be linked to the higher incidence of comorbidities (heart failure in particular) and less favourable haemodynamic and haemostatic profiles, which most likely determine the course of the acute stage of stroke [19, 20]. Reduced cardiac output and low cerebral blood flow, which could contribute to impaired cerebral autoregulation, or underdeveloped cerebral collateral circulation, are other postulated mechanisms [21]. Atrial flutter has many clinical aspects that are similar to those of AF (e.g. underlying disease, predisposing factors, complications, and medical management); however, the diverse influences on the haemodynamics and probably the coagulation parameter differences between these two arrhythmias may lead to different stroke distributions and prognoses. Transoesophageal echocardiography studies have demonstrated that atrial mechanical dysfunction is more pronounced in AF than in AFl [22]. We showed that patients with AFl had higher mean LVEFs and less frequent chronic heart failure than patients with AF. It has been documented that patients with AF and concomitant heart failure with either preserved or reduced LVEFs suffer from worse prognoses, including increased mortality [1]. We showed that AF is associated with poor outcomes after adjusting for the baseline neurological status (mRS) and stroke aetiology; thus, it seems unlikely that the reduced cerebral blood flow is a major determinant of the outcome. However, the design of this study did not enable us to conduct detailed electrographic or echocardiographic investigations to clarify this issue.
Interestingly, there have been some observations that subjects with AF have frequent periventricular white matter lesions and that AF is associated with impairment of endothelial dysfunction, which is reversed by the restoration of the sinus rhythm [23, 24]. These data are interesting because they suggest that AF might affect stroke severity and poststroke outcomes via concomitant or resultant endothelial dysfunction, oxidative stress, and inflammation. The functional and structural changes in the atrial myocardium and the stasis of blood in the left atrial appendage generate a prothrombotic state in AF and probably AFl; however, whether inflammatory states and the systemic activation of the coagulatory system influence the course of cardioembolic and non-cardioembolic stroke, and whether these factors differ between AF and AFl, remain unknown [25]. Although we based the stroke diagnosis on computed tomography (CT) scans, future trials should evaluate radiologic embolic stroke features based on magnetic resonance imaging (MRI) examinations because the characteristic lesion patterns in these patients might provide the missing link between the pathophysiologies of AFl anAlthough the optimal management of AFl without a known history of AF is uncertain, patients with AFl should be considered for long-term anticoagulation in a manner similar to those with AF, because AFl carries a risk for systemic embolisation, and these patients usually have episodes of AF [3]. However, a recently published national cohort study conducted in Taiwan demonstrated that solitary AFl patients (in isolation without AF) who had not received anticoagulation therapy had a lower risk of stroke or systemic embolisation than AFl patients developing AF, and anticoagulation therapy was most effective in the patients with solitary AFl who had a high embolic risk (CHA2DS2-VASc ≥ 3) [26]. Similar to other studies, we showed that nearly half of the patients with known AA and those with AFl admitted due to CVA had not received chronic anticoagulation therapy, suggesting a substantial need to improve stroke prophylaxis in high-risk patients with AF and AFl [27]. Additionally, the failure to use adequate chronic anticoagulation could not only have increased the cardioembolic risk but also had a negative impact on the stroke prognosis independent of the presence of AFl or AF, as demonstrated in the multivariable analysis.
Competing causes of IS/TIA in AF patients, such as carotid atherosclerosis and/or cerebral small vessel disease, have been reported in approximately 25% of cases [28]. We also demonstrated that competing causes are even more common in AFl, because 56% of the patients with AFl in our cohort had more than one evident mechanism of IS. Some studies have demonstrated that patients with AF who suffered a stroke despite therapeutic anticoagulation were more often smokers and had hypertension and dyslipidaemia, and these results are consistent with our data [29]. Secondary stroke prophylactics in patients who experienced ischaemic stroke on therapeutic anticoagulation is not well established. The combination of an anticoagulant and an antiaggregant has thus far not been justified, due to the significant risk of intracerebral haemorrhage, but it seems increasingly important to modify the lifestyle of patients, including dietary treatment, smoking cessation, and, if possible, increasing physical activity.
Our study demonstrated higher (5.8% vs. 2%) than previously reported percentages of AFl in patients with IS/TIA, which was probably caused by the differences in the selection of patients compared with earlier analyses (e.g. no differentiation between ischaemic and haemorrhagic strokes and exclusion of patients with valvular AF or TIA), use of 24-h Holter ECGs on most patients, and inclusion of all IS stroke patients regardless of age [30–32]. However, we cannot exclude the omission of patients with asymptomatic paroxysmal AFl with rare arrhythmic events or the coexistence of paroxysmal AFl with AF, which is often difficult to detected by standard ECG and Holter ECG. In our cohort, only 3 patients had paroxysmal AFl (0.4% of patients with AA). However, this type of AA is associated with a low risk of IS [10]. Due to the different AF definitions, there are also many discrepancies in the reported incidence of AFl and individual types of AF in patients with CVA.
Several limitations of our study merit consideration. This analysis was limited by its retrospective cross-sectional design involving a single centre and substantial disproportion between the study groups, resulting in a large confidence interval in the multivariable analysis; thus, our findings should be considered hypothesis-generating, and prospective studies focusing on this topic are warranted. Another limitation is that isolated, long-term AFl is a rare finding, and many patients with AFl have alternating periods of AF; thus, determining the exact risk of embolisation and course of stroke attributable to AA is challenging. In a large cohort of patients with AFl without known previous AF, over a follow-up duration of 3 years, 40.4% of subjects developed AF compared with 3.3% of the matched general population (risk ratio, 12.2; p < 0.001) [1]. Additionally, the prevalence of AF before catheter ablation of AFl in the current literature varies from 24% to 62% [33]. Studies have demonstrated a close correlation between a history of previous embolism and periods of AF during atrial flutter, and revealed that among patients who did not have a history of AF, left atrial thrombus or spontaneous left atrial echo contrast were found in 1% to 1.5% and 11% to 13% of patients, respectively [34, 35]. At our centre, it is the standard of care to perform Holter monitoring on all stroke patients with no apparent AF on ECG or inpatient rhythm monitoring (regardless of the presence or absence of alternative stroke pathogeneses); however, some patients were unable to undergo Holter monitoring for various reasons, such as early mortality. We did not include patients with PAF because we suspected that the pattern of AF (paroxysmal vs. permanent/persistent AF) may influence the results, and our suspicion was recently confirmed in the ENGAGE AF-TIMI 48 trial in which there were fewer deaths among patients with PAF than among those with persistent and permanent AF [36]. We did not analyse permanent and persistent AF separately, because the classification does not depend on the pathophysiology or clinical characteristics of arrhythmia [37]. Another limitation is that other potential confounders, such antiarrhythmic drug use, the impact of nontherapeutic international normalized ratio (INR 1.6–1.9), and adherence to antihypertensive and lipid-lowering drugs, were not assessed.
However, our study had some strengths. We analysed a large group of patients with AA and used the recommended CCS classification system to discriminate the phenotypic stroke subtypes. To the best of our knowledge, the course of stroke in AFl and the association between AFl and non-cardioembolic events, mainly lacunar strokes, have not been previously studied or reported. Further studies are needed to clarify the causative role of AFl during IS and how these cases should be managed for optimal stroke prevention. The fundamental goals of the accurate classification of the IS subcategory are to make a correct diagnosis, enable prompt secondary preventative treatment, and predict the risk of future recurrence. The current guidelines regarding AF do not emphasise global risk-factor management, and a comprehensive approach to stroke prevention should explore and emphasise the intensive management of all risk factors rather than only focusing on recommendations regarding anticoagulant therapy [38].
In conclusion, the clinical course of ischaemic stroke in AFl and AF varies. Disabling or fatal IS was observed less frequently in patients with AFl than in patients with permanent AF. This finding can possibly be explained by the more frequent occurrence of lacunar strokes in AFl patients. Atrial flutter was observed in only 5.8% of patients hospitalised due to acute cerebrovascular accidents, confirming that this type of AF is a rare arrythmia in stroke patients. Our results also emphasise the importance of proper prophylactic anticoagulation and strict control of atherothrombotic vascular risk factors in patients with AF or AFl.