Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
NEUROLOGY / EXPERIMENTAL RESEARCH
Celastrol attenuates Alzheimer’s disease-mediated learning and memory impairment by inhibiting endoplasmic reticulum stress-induced inflammation and oxidative stress
1
School of Gongli Hospital Medical Technology, University of Shanghai for Science and Technology, Shanghai, China
2
Shanghai Health Commission Key Lab of Artificial Intelligence (AI)-Based Management of Inflammation and Chronic Diseases, Department of Central Laboratory, Gongli Hospital of Shanghai Pudong New Area, Shanghai, China
3
Department of Clinical Laboratory, Gongli Hospital of Shanghai Pudong New Area, Shanghai, China
4
Department of Ultrasound, Gongli Hospital of Shanghai Pudong New Area, Shanghai, China
Submission date: 2024-03-04
Final revision date: 2024-05-27
Acceptance date: 2024-06-11
Online publication date: 2024-06-12
Corresponding author
Qiuyun Liu
Department of Ultrasound Gongli Hospital of Shanghai Pudong New Area No. 207, Juye Rd. Pudong New District Shanghai, 200135, China
Department of Ultrasound Gongli Hospital of Shanghai Pudong New Area No. 207, Juye Rd. Pudong New District Shanghai, 200135, China
Denghai Zhang
Shanghai Health Commission Key Lab of Artificial Intelligence (AI)-Based Management of Inflammation and Chronic Diseases Department of Central Laboratory Gongli Hospital of Shanghai Pudong New Area No. 207, Juye Rd. Pudong New District, Shanghai 200135, China Phone: +86 18916173857
Shanghai Health Commission Key Lab of Artificial Intelligence (AI)-Based Management of Inflammation and Chronic Diseases Department of Central Laboratory Gongli Hospital of Shanghai Pudong New Area No. 207, Juye Rd. Pudong New District, Shanghai 200135, China Phone: +86 18916173857
KEYWORDS
TOPICS
ABSTRACT
Introduction:
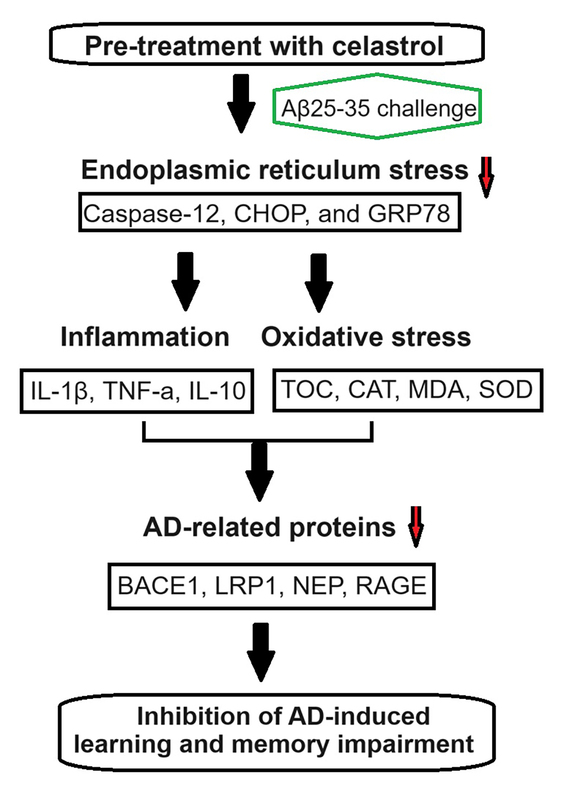
Alzheimer’s disease (AD) is triggered by biological mechanisms such as neuroinflammation and oxidative stress. Endoplasmic reticulum (ER) stress can lead to the expression of molecular chaperones in the ER, which helps in restoring cellular homeostasis. Researchers have highlighted the role of ER stress in the progression of AD, suggesting that regulating it could be a potential treatment strategy for AD.
Material and methods:
We induced AD in mice by injecting amyloid beta-peptide 25-35 (Aβ25-35) bilaterally into the CA1 of the dorsal hippocampus. Some mice were administered celastrol intraperitoneally before the Aβ25-35 injection, while others received it after the injection. The mice underwent the Barnes maze cognitive test and Morris water maze test to assess learning and memory impairment. Levels of interleukin (IL)-1β, tumor necrosis factor α, and IL-10 were measured to evaluate inflammation, while total antioxidant capacity, catalase, malondialdehyde, and superoxide dismutase levels were analyzed to estimate oxidative stress.
Results:
Our study showed that pre-treatment with celastrol could prevent learning and memory decline in AD mice by reducing inflammation and oxidative stress. Celastrol also inhibited AD-induced inflammation and oxidative stress. Additionally, celastrol suppressed AD progression by targeting ER stress. These results suggest that celastrol treatment could be beneficial in addressing learning and memory deficits in AD, paving the way for potential neuroprotective treatments.
Conclusions:
Celastrol effectively improved learning and memory impairments in AD mice by targeting ER stress-induced inflammation and oxidative stress. This highlights the potential of celastrol as a therapeutic agent for AD.
Alzheimer’s disease (AD) is triggered by biological mechanisms such as neuroinflammation and oxidative stress. Endoplasmic reticulum (ER) stress can lead to the expression of molecular chaperones in the ER, which helps in restoring cellular homeostasis. Researchers have highlighted the role of ER stress in the progression of AD, suggesting that regulating it could be a potential treatment strategy for AD.
Material and methods:
We induced AD in mice by injecting amyloid beta-peptide 25-35 (Aβ25-35) bilaterally into the CA1 of the dorsal hippocampus. Some mice were administered celastrol intraperitoneally before the Aβ25-35 injection, while others received it after the injection. The mice underwent the Barnes maze cognitive test and Morris water maze test to assess learning and memory impairment. Levels of interleukin (IL)-1β, tumor necrosis factor α, and IL-10 were measured to evaluate inflammation, while total antioxidant capacity, catalase, malondialdehyde, and superoxide dismutase levels were analyzed to estimate oxidative stress.
Results:
Our study showed that pre-treatment with celastrol could prevent learning and memory decline in AD mice by reducing inflammation and oxidative stress. Celastrol also inhibited AD-induced inflammation and oxidative stress. Additionally, celastrol suppressed AD progression by targeting ER stress. These results suggest that celastrol treatment could be beneficial in addressing learning and memory deficits in AD, paving the way for potential neuroprotective treatments.
Conclusions:
Celastrol effectively improved learning and memory impairments in AD mice by targeting ER stress-induced inflammation and oxidative stress. This highlights the potential of celastrol as a therapeutic agent for AD.
REFERENCES (54)
1.
Sun C, Liu J, Duan F, Cong L, Qi X. The role of the microRNA regulatory network in Alzheimer’s disease: a bioinformatics analysis. Arch Med Sci 2021; 18: 206-22.
2.
Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 2020; 25: 5789.
3.
Ali H, Tofrizal T, Tjong DH, Yanis A, Yarni SD. The RYR3 gene is involved in Wharton’s jelly derived mesenchymal stem cell treatment of Alzheimer’s disease in rats. Arch Med Sci 2023; 19: 820-4.
4.
Zhang N, Xu H, Wang Y, et al. Protective mechanism of kaempferol against A25-35-mediated apoptosis of pheochromocytoma (PC-12) cells through the ER/ERK/MAPK signalling pathway. Arch Med Sci 2020; 17: 406-16.
5.
Ma C, Hong F, Yang S. Amyloidosis in Alzheimer’s disease: pathogeny, etiology, and related therapeutic directions. Molecules 2022; 27: 1210.
6.
Papuć E, Rejdak K. The role of myelin damage in Alzheimer’s disease pathology. Arch Med Sci 2018; 16: 345-51.
7.
Flirski M, Sobow T, Kloszewska I. Behavioural genetics of Alzheimer’s disease: a comprehensive review. Arch Med Sci 2011; 7: 195-210.
8.
Ng A, Tam WW, Zhang MW, et al. IL-1, IL-6, TNF- and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and Meta-analysis. Sci Rep 2018; 8: 12050.
9.
Alhowail AH. Pioglitazone ameliorates DOX-induced cognitive impairment by mitigating inflammation, oxidative stress, and apoptosis of hippocampal neurons in rats. Behav Brain Res 2024; 457: 114714.
10.
Juszczyk G, Mikulska J, Kasperek K, et al. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer’s disease: the role of antioxidants in prevention and treatment. Antioxidants 2021; 10: 1439.
11.
Singh A, Kukreti R, Saso L, et al. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 2019; 24: 1583.
12.
Jiang S, Yu LJ, Yang H, et al. A study on inhibition of the A1-42-induced inflammatory response by the Huatuo Zaizao pill through the NF-κB signaling pathway. Arch Med Sci 2020; 19: 1136-44.
13.
Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging 2018; 13: 757-72.
14.
Chen X, Zhang B, Li J, et al. Celastrol attenuates incision-induced inflammation and pain associated with inhibition of the NF-kappaB signalling pathway via SARM. Life Sci 2018; 205: 136-44.
15.
Kim Y, Kang H, Jang SW, et al. Celastrol inhibits breast cancer cell invasion via suppression of NF-kB-mediated matrix metalloproteinase-9 expression. Cell Physiol Biochem 2011; 28: 175-84.
16.
Venkatesha SH, Dudics S, Astry B, et al. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Patho Dis 2016; 74: ftw059.
17.
Luo D, Guo Y, Cheng Y, et al. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-kappaB pathways. Aging 2017; 9: 2069-82.
18.
Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 2016; 73: 79-94.
19.
Groenendyk J, Agellon LB, Michalak M. Calcium signaling and endoplasmic reticulum stress. Int Rev Cell Mol Biol 2021; 363: 1-20.
20.
Ajoolabady A, Lindholm D, Ren J, et al. ER stress and UPR in Alzheimer’s disease: mechanisms, pathogenesis, treatments. Cell Death Dis 2022; 13: 706.
21.
Li X, Qin Y, Ye S, et al. Protective effect of Huangpu Tongqiao capsule against Alzheimer’s disease through inhibiting the apoptosis pathway mediated by endoplasmic reticulum stress in vitro and in vivo. Saudi Pharm J 2022; 30: 1561-71.
22.
Kou JJ, Shi JZ, He YY, et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol Sin 2022; 43: 840-9.
23.
Lu W, Huang J, Sun S, et al. Changes in lactate content and monocarboxylate transporter 2 expression in Abeta(2)(5)(-)(3)(5)-treated rat model of Alzheimer’s disease. Neurol Sci 2015; 36: 871-6.
24.
Benveniste H, Jørgensen MB, Sandberg M, et al. Ischemic damage in hippocampal CA1 is dependent on glutamate release and intact innervation from CA3. J Cereb Blood Flow Metab 1989; 9: 629-39.
25.
Chen M, Liu M, Luo Y, et al. Celastrol protects against cerebral ischemia/reperfusion injury in mice by inhibiting glycolysis through targeting HIF-1/PDK1 axis. Oxid Med Cell Longev 2022; 2022: 7420507.
26.
Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neuroscience 2014; 261: 1-10.
27.
Fang X, Li S, Han Q, et al. Overexpression cdc42 attenuates isoflurane-induced neurotoxicity in developmental brain of rats. Biochem Biophys Res Commun 2017; 490: 719-25.
28.
Zhang B, Chen X, Lv Y, et al. Cdh1 overexpression improves emotion and cognitive-related behaviors via regulating hippocampal neuroplasticity in global cerebral ischemia rats. Neurochem Int 2019; 124: 225-37.
29.
Kang MG, Lee HJ, Cho JY, et al. Anti-inflammatory effects of sucrose-derived oligosaccharides produced by a constitutive mutant L. mesenteroides B-512FMCM dextransucrase in high fat diet-fed mice. Biochem Biophys Res Commun 2016; 477: 350-5.
30.
Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388-405.
31.
Gurel A, Coskun O, Armutcu F, et al. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat 2005; 29: 173-8.
32.
Winterbourn CC, Gutteridge JM, Halliwell B. Doxorubicin-dependent lipid peroxidation at low partial pressures of O2. J Free Radic Biol Med 1985; 1: 43-9.
33.
Benham AM. Protein folding and disulfide bond formation in the eukaryotic cell: meeting report based on the presentations at the European Network Meeting on Protein Folding and Disulfide Bond Formation 2009 (Elsinore, Denmark). FEBS J 2009; 276: 6905-11.
34.
Kato H, Nishitoh H. Stress responses from the endoplasmic reticulum in cancer. Front Oncol 2015; 5: 93.
35.
Schapansky J, Morissette M, Odero G, et al. Neuregulin beta1 enhances peak glutamate-induced intracellular calcium levels through endoplasmic reticulum calcium release in cultured hippocampal neurons. Can J Physiol Pharmacol 2009; 87: 883-91.
36.
Mandl J, Mészáros T, Bánhegyi G, et al. Minireview: endoplasmic reticulum stress: control in protein, lipid, and signal homeostasis. Mol Endocrinol 2013; 27: 384-93.
37.
Nagar P, Sharma P, Dhapola R, et al. Endoplasmic reticulum stress in Alzheimer’s disease: molecular mechanisms and therapeutic prospects. Life Sci 2023; 330: 121983.
38.
2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023; 19: 1598-695.
39.
Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet 2021; 397: 1577-90.
40.
Babazadeh A, Mohseni Afshar Z, Javanian M, et al. Influenza vaccination and Guillain-Barre syndrome: reality or fear. J Transl Int Med 2019; 7: 137-42.
41.
Zhao Y, Zhao H, Lobo N, et al. Celastrol enhances cell viability and inhibits amyloid-beta production induced by lipopolysaccharide in vitro. J Alzheimers Dis 2014; 41: 835-44.
42.
Jiang M, Liu X, Zhang D, et al. Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J Neuroinflamm 2018; 15: 78.
43.
Tang M, Cao X, Zhang K, et al. Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis 2018; 9: 601.
44.
Patel S, Bansoad AV, Singh R, Khatik GL. BACE1: a key regulator in Alzheimer’s disease progression and current development of its inhibitors. Curr Neuropharmacol 2022; 20: 1174-93.
45.
Shinohara M, Tachibana M, Kanekiyo T, Bu G. Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies. J Lipid Res 2017; 58: 1267-81.
46.
Qian C, Yang C, Lu M, et al. Activating AhR alleviates cognitive deficits of Alzheimer’s disease model mice by upregulating endogenous A catabolic enzyme Neprilysin. Theranostics 2021; 11: 8797-812.
47.
Turrel O, Goguel V, Preat T. Drosophila Neprilysin 1 rescues memory deficits caused by amyloid- peptide. J Neurosci 2017; 37: 10334-45.
48.
Klein C, Roussel G, Brun S, et al. 5-HIAA induces neprilysin to ameliorate pathophysiology and symptoms in a mouse model for Alzheimer’s disease. Acta Neuropathol Commun 2018; 6: 136.
49.
Guan L, Mao Z, Yang S, et al. Dioscin alleviates Alzheimer’s disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed Pharmacother 2022; 152: 113248.
50.
Huang G, Zang J, He L, et al. Bioactive nanoenzyme reverses oxidative damage and endoplasmic reticulum stress in neurons under ischemic stroke. ACS Nano 2022; 16: 431-52.
51.
Chen X, Mi L, Gu G, et al. Dysfunctional endoplasmic reticulum-mitochondrion coupling is associated with endoplasmic reticulum stress-induced apoptosis and neurological deficits in a rodent model of severe head injury. J Neurotrauma 2022; 39: 560-76.
52.
Hoozemans JJ, Veerhuis R, Van Haastert ES, et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol 2005; 110: 165-72.
53.
Costa RO, Ferreiro E, Martins I, et al. Amyloid -induced ER stress is enhanced under mitochondrial dysfunction conditions. Neurobiol Aging 2012; 33: 824.e5-e16.
54.
Zhang Q, Liu J, Chen S, et al. Caspase-12 is involved in stretch-induced apoptosis mediated endoplasmic reticulum stress. Apoptosis 2016; 21: 432-42.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.