Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
Causal association between vitamin D levels and neonatal jaundice: results from a Mendelian randomization study
1
Department of Medical Records Statistics, Wujin Hospital Affiliated with Jiangsu University, Changzhou, China
2
The Wujin Clinical College of Xuzhou Medical University, Changzhou, China
3
Department of Quality Control, Wujin Hospital Affiliated with Jiangsu University, Changzhou, China
Submission date: 2024-05-30
Final revision date: 2024-08-18
Acceptance date: 2024-08-22
Online publication date: 2024-10-16
Corresponding author
Li-Wen Wang
Department of Medical Quality Control, Wujin Hospital Affiliated to Jiangsu University 2 Yongning North Road Tianning District Changzhou, Jiangsu 213000, China, Phone: +86 15951228866
Department of Medical Quality Control, Wujin Hospital Affiliated to Jiangsu University 2 Yongning North Road Tianning District Changzhou, Jiangsu 213000, China, Phone: +86 15951228866
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Observational studies have suggested an association between vitamin D deficiency and the risk of neonatal jaundice; however, it remains unclear whether this relationship is causal. We conducted a Mendelian randomization (MR) study to evaluate whether vitamin D levels influence the risk of neonatal jaundice.
Material and methods:
Single nucleotide polymorphisms (SNPs) highly associated with vitamin D levels were selected as instrumental variables from publicly available genome-wide association studies (GWAS). MR analysis was conducted using five different models, including MR-Egger regression and inverse-variance weighting (IVW). Sensitivity analysis included MR-PRESSO (MR-pleiotropy residual sum and outlier) testing, Cochran’s Q heterogeneity testing, the MR-Egger method, leave-one-out analysis, and Bayesian colocalization analysis to determine whether there were shared causal loci between vitamin D levels and neonatal jaundice.
Results:
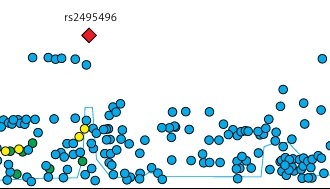
A total of 160 SNPs with genome-wide significance for vitamin D levels were identified, explaining 1.4% of the genetic variance in vitamin D levels. The MR-PRESSO test did not detect any outlier values, and heterogeneity testing did not identify significant heterogeneity. However, pleiotropy testing revealed significant horizontal pleiotropy, prompting the use of the MR-Egger regression model for MR analysis. The results indicated a significant negative causal association between vitamin D levels and the risk of neonatal jaundice (OR = 0.04, 95% CI: 0.004–0.43, p = 0.0026). Sensitivity analysis and colocalization analysis further confirmed the accuracy and robustness of the results.
Conclusions:
Genetically reduced vitamin D levels are causally associated with an increased risk of neonatal jaundice.
Observational studies have suggested an association between vitamin D deficiency and the risk of neonatal jaundice; however, it remains unclear whether this relationship is causal. We conducted a Mendelian randomization (MR) study to evaluate whether vitamin D levels influence the risk of neonatal jaundice.
Material and methods:
Single nucleotide polymorphisms (SNPs) highly associated with vitamin D levels were selected as instrumental variables from publicly available genome-wide association studies (GWAS). MR analysis was conducted using five different models, including MR-Egger regression and inverse-variance weighting (IVW). Sensitivity analysis included MR-PRESSO (MR-pleiotropy residual sum and outlier) testing, Cochran’s Q heterogeneity testing, the MR-Egger method, leave-one-out analysis, and Bayesian colocalization analysis to determine whether there were shared causal loci between vitamin D levels and neonatal jaundice.
Results:
A total of 160 SNPs with genome-wide significance for vitamin D levels were identified, explaining 1.4% of the genetic variance in vitamin D levels. The MR-PRESSO test did not detect any outlier values, and heterogeneity testing did not identify significant heterogeneity. However, pleiotropy testing revealed significant horizontal pleiotropy, prompting the use of the MR-Egger regression model for MR analysis. The results indicated a significant negative causal association between vitamin D levels and the risk of neonatal jaundice (OR = 0.04, 95% CI: 0.004–0.43, p = 0.0026). Sensitivity analysis and colocalization analysis further confirmed the accuracy and robustness of the results.
Conclusions:
Genetically reduced vitamin D levels are causally associated with an increased risk of neonatal jaundice.
REFERENCES (28)
1.
Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health 2018; 2: 610-20.
2.
van Der Geest BAM, de Mol MJS, Barendse ISA, et al. Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study. Sci Rep 2022; 12: 14385.
3.
Qian S, Kumar P, Testai FD. Bilirubin encephalopathy. Curr Neurol Neurosci Rep 2022; 22: 343-53.
4.
Alkén J, Håkansson S, Ekéus C, Gustafson P, Norman M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw Open 2019; 2: e190858.
5.
Janoušek J, Pilařová V, Macáková K, et al. Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit Rev Clin Lab Sci 2022; 59: 517-54.
6.
Ismailova A, White JH. Vitamin D. Infections and immunity. Rev Endocr Metab Disord 2022; 23: 265-77.
7.
Saponaro F, Saba A, Zucchi R. An update on vitamin D metabolism. Int J Mol Sci 2020; 21: 6573.
8.
Hansen TWR, Wong RJ, Stevenson DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev 2020; 100: 1291-346.
9.
Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J 2023; 44: 4913-24.
10.
Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 2016; 27: 3253-65.
11.
Paz V, Dashti HS, Burgess S, Garfield V. Selection of genetic instruments in Mendelian randomisation studies of sleep traits. Sleep Med 2023; 112: 342-51.
12.
Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res 2017; 26: 2333-55.
13.
Yang Q, Sanderson E, Tilling K, Borges MC, Lawlor DA. Exploring and mitigating potential bias when genetic instrumental variables are associated with multiple non-exposure traits in Mendelian randomization. Eur J Epidemiol 2022; 37: 683-700.
14.
Zuber V, Grinberg NF, Gill D, et al. Combining evidence from Mendelian randomization and colocalization: review and comparison of approaches. Am J Hum Genet 2022; 109: 767-82.
15.
Chen J, Xu F, Ruan X, et al. Therapeutic targets for inflammatory bowel disease: proteome-wide Mendelian randomization and colocalization analyses. EBioMedicine 2023; 89: 104494.
16.
Aletayeb SM, Dehdashtiyan M, Aminzadeh M, Malekyan A, Jafrasteh S. Comparison between maternal and neonatal serum vitamin D levels in term jaundiced and nonjaundiced cases. J Chin Med Assoc 2016; 79: 614-7.
17.
Le J, Yuan TF, Geng JQ, Wang ST, Li Y, Zhang BH. Acylation Derivatization based LC-MS analysis of 25-hydroxyvitamin D from finger-prick blood. J Lipid Res 2019; 60: 1058-64.
18.
Huang J, Zhao Q, Li J, et al. Correlation between neonatal hyperbilirubinemia and vitamin D levels: a meta-analysis. PLoS One 2021; 16: e0251584.
19.
Fernando M, Coster TG, Ellery SJ, et al. Relationships between total, free and bioavailable vitamin D and vitamin D binding protein in early pregnancy with neonatal outcomes: a retrospective cohort study. Nutrients 2020; 12: 2495.
20.
Wang L, Zhang C, Song Y, Zhang Z. Serum vitamin D deficiency and risk of gestational diabetes mellitus: a meta-analysis. Arch Med Sci 2020; 16: 742-51.
21.
Wu C, Song Y, Wang X. Vitamin D supplementation for the outcomes of patients with gestational diabetes mellitus and neonates: a meta-analysis and systematic review. Int J Clin Pract 2023; 2023: 1907222.
22.
Doan TNK, Vo DK, Kim H, et al. Differential effects of 1,25-dihydroxyvitamin D3 on the expressions and functions of hepatic CYP and UGT enzymes and its pharmacokinetic consequences in vivo. Pharmaceutics 2020; 12: 1129.
23.
Kaplan M, Hammerman C, Rubaltelli FF, et al. Hemolysis and bilirubin conjugation in association with UDP-glucuronosyltransferase 1A1 promoter polymorphism. Hepatology 2002; 35: 905-11.
24.
Cojic M, Kocic R, Klisic A, et al. A novel mechanism of vitamin D anti-inflammatory/antioxidative potential in type 2 diabetic patients on metformin therapy. Arch Med Sci 2020; 16: 1004-12.
25.
Barchetta I, Cimini FA, Cavallo MG. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): an update. Nutrients 2020; 12: 3302.
26.
Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The role of vitamin D and vitamin D binding protein in chronic liver diseases. Int J Mol Sci 2022; 23: 10705.
27.
Tavakoli H, Rostami H, Avan A, et al. High dose vitamin D supplementation is associated with an improvement in serum markers of liver function. Biofactors 2019; 45: 335-42.
28.
Zhou W, Wang P, Bai Y, Zhang Y, Shu J, Liu Y. Vitamin D metabolic pathway genes polymorphisms and vitamin D levels in association with neonatal hyperbilirubinemia in China: a single-center retrospective cohort study. BMC Pediatr 2023; 23: 275.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.