Systemic lupus erythematosus (SLE) is a common systemic autoimmune disease characterized by a high expression of autoantibodies and the systemic involvement of various systems, with different clinical manifestations and complex pathogenesis. The prevalence of SLE is approximately 12/105–39/105, and this condition affects 90% of women of childbearing age [1]. In general, SLE can develop at any age. However, there is a significant difference in terms of sex. The female-to-male (F : M) ratio is approximately 1.2 in children aged under 10 years, and it increases to 8–15 in child-bearing individuals and decreases to approximately 3 in elderly people [2]. Therefore, there is a significant dynamic change in the F : M ratio with age. SLE commonly develops during adolescence or in the old age period, and middle-age onset is relatively rare.
Neuropsychiatric systemic lupus erythematosus (NPSLE) comprises a group of complications with poor prognosis and high mortality caused by SLE-related neurological or psychiatric symptoms. Furthermore, it has diverse clinical manifestations, which primarily include central and peripheral nervous system symptoms [3]. NPSLE indicates the activity of SLE lesions, and it is commonly associated with central nervous system symptoms. In contrast, peripheral nervous system symptoms, particularly facial palsy (FP) caused by SLE-related peripheral neurological lesions, are relatively rare [4]. Herein, we report a case of SLE in a middle-aged male patient who presented with FP at onset. To the best of our knowledge, this is the first case of such a clinical manifestation.
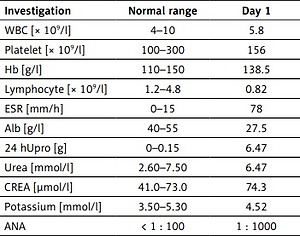
A 43-year-old male patient who presented with distortion of commissure for 1 week was admitted to the nephrology department of our hospital on 9 December 2016. Urinalysis revealed a protein level of 3+. The patient was previously fit, and physical examination upon admission revealed the following results: temperature 36.2°C, blood pressure 160/110 mm Hg, the disappearance of bilateral forehead wrinkles, shallow bilateral nasolabial groove (which is evident on the right side), inability to blow air, tongue extension in the middle, soft neck, normal muscle strength and tone in the extremities, and pitting oedema in both lower extremities. The results of the main laboratory examination in our hospital are shown in Table I. Furthermore, the antiphospholipid antibody, IgA, IgG, IgM, and C-reactive protein levels were normal. Chest radiography, electrocardiogram, cardiac, abdominal, and urological ultrasonography, cranial computed tomography (CT) scan, and magnetic resonance imaging (MRI) did not show any significant abnormalities. The patient was then diagnosed with FP by a neurologist, and he received nutritional nerve therapy and local physiotherapy. Subsequent rheumatology consultation indicated SLE-lupus nephritis (LN). Finally, the patient was diagnosed with SLE, LN, and FP. Renal biopsy (diffusely and proliferatively glomerular nephritis) revealed class IV LN. Methylprednisolone (48 mg once daily), compounded cyclophosphamide (50 mg twice daily), and hydroxychloroquine sulphate (0.1 g 3 times daily) were administered. The patient’s FP symptoms had completely resolved after 4 weeks. A follow-up re-examination suggested a significant reduction in 24-h urine protein level, an increase in plasma albumin level, and a remarkable improvement in general condition. The patient is currently undergoing regular long-term follow-up, and his condition is stable.
Table I
Laboratory examination in our hospital
[i] WBC – white blood cell, Hb – haemoglobin, ESR – erythrocyte sedimentation rate, Alb – albumin, 24 hUpro – 24-hour urine proteins, CREA – creatinine, ANA – anti-nuclear antibody, anti-RNP – anti-nuclear ribonucleoprotein antibody, anti-Sm – anti-Smith antibody, C 3 – complement 3, C 4 – complement 4, ASO – anti streptolysin O, RF – rheumatoid factor, AGBM – anti-glomerular basement membrane antibody, ANCA – anti-neutrophil cytoplasmic antibody.
In the current case, the following clinical characteristics were observed: The patient had acute onset, with distortion of commissure, which is the primary onset symptom. The patient was then diagnosed with FP, a clinical manifestation of peripheral neuropathy, by a neurologist. Then, biochemical tests upon admission showed significant proteinuria, hypoalbuminaemia, and clinical manifestations of nephrotic syndrome. Routine blood tests revealed a low total lymphocyte count. Immunological tests showed hypocomplementemia, which was represented by low C3 and C4 levels. Furthermore, the patient tested positive for the anti-nuclear antibody (ANA), anti-Smith antibody (Sm), and anti-nuclear ribonucleoprotein antibody (RNP). Elective renal biopsy revealed class IV LN. According to the clinical diagnostic criteria of SLE, the patient was diagnosed with SLE, LN, and FP.
In this case, the patient initially presented with FP, which is the clinical manifestation of peripheral neuropathy in NPSLE. There are no specific diagnostic criteria for the clinical diagnosis of NPSLE, thereby making it difficult to distinguish from other neurological diseases. Therefore, the condition can only be diagnosed in combination with other relevant ancillary tests [5]. The current guidelines recommend a cranial CT scan and MRI for the diagnosis of NSPLE. In particular, MRI, which is an important procedure, can be performed repeatedly if necessary [6]. Based on the previous physical examination, there was no history of cerebrovascular disease, and both cranial CT scan and MRI results upon admission were normal. Therefore, the clinical diagnosis of NPSLE was definite. The differential diagnosis of NPLSE should exclude central nervous system infection, reversible posterior lesion syndrome, and metabolism encephalopathy. Above all, exclusion of central nervous system infections is crucial in the treatment of SLE, because the principles of treatment are polar opposites for them. In conclusion, the diagnosis of NPLES is often established on the basis of the exclusion of differential diagnosis in clinical practice [5]. Hence, the diagnosis of NPSLE crucially depends on the history, clinical symptoms, and response to treatment of these patients. For the treatment of SLE, there are not only traditional glucocorticoids and immunosuppressants, but also some new agents including biologics that target B cells, T cells and type 1 interferons, and small molecules that restrain kinases. Among them, belimumab was the first biologic created exclusively for SLE and meeting its primary end aim [1].
The incidence of SLE is significantly higher in women than in men. LN, one of the most common complications of SLE, has a clear sex predominance, and it primarily affects adult women. Sex differences indicate that the onset of LN may be correlated with oestrogen levels. However, several studies have reported regional and ethnic differences in terms of age at peak onset in men with LN [7–10]. Type IV LN is the most common pathological type in both male and female patients. In the current case, the patient developed LN, which is a rare manifestation. However, the pathological diagnosis of nephropathy was class IV LN, which is consistent with the finding of a previous study [10].
With respect to sex differences in serological results among individuals, male patients with SLE commonly test positive for anti-dsDNA antibodies, and they have high anticardiolipin antibodies and low complement C3 levels. Meanwhile, female patients with SLE usually present with low total leukocyte and lymphocyte levels [11, 12]. In our case, the patient had low C3 and C4 levels and total lymphocyte levels in the blood. Also, he tested positive for ANA, Sm, and RNP. These findings were partly consistent with those previously mentioned.
The principles of SLE treatment are generally similar in men and women. However, there are differences in several aspects. In general, men with SLE are more commonly hospitalized for longer periods than women, and they frequently develop lesions in the kidneys and nervous system. In addition, male patients usually require immunosuppressive agents, biologics, and replacement therapy. The prognosis of male patients is relatively poor due to high SLE severity [12, 13]. In our case, after triple therapy with oral glucocorticoids, cyclophosphamide, and hydroxychloroquine, the patient’s FP symptoms gradually resolved, and his nephropathy improved significantly. During long-term follow-up after discharge, the patient’s condition was stable. Moreover, infection, gastrointestinal bleeding, or femoral head necrosis, which can be caused by the long-term application of glucocorticoids and immunosuppressants, was not observed. Hence, the treatment was safe and effective. However, the long-term effect of this regimen must be further investigated.
The aetiology and pathogenesis of SLE in men are complex, and the clinical presentation is not specific. Recent research has found that IL-31 and IL-33 may be novel biomarkers and future therapeutic targets for immunological disease, including SLE [14]. Moreover, when treating SLE, TNF-inhibitors can be used either alone or in conjunction with other anti-inflammatory medications [15]. To reduce the risk of infection in treatment, proper immunizations against influenza, pneumoniae, and HBV should be given [16].
Despite early diagnosis and treatment, the morbidity and mortality rates of SLE among men are high, although the diagnosis rate is low overall. Currently, some potential issues regarding the diagnosis and management of SLE in men need to be addressed. Therefore, an in-depth study on the characteristics of SLE in men should be performed to facilitate a timely and accurate diagnosis, thereby leading to more effective treatment and management schemes [17].