Introduction
Multiple sclerosis (MS) belongs to the group of chronic autoimmune inflammatory diseases of the central nervous system. Morphologically, it is characterised by inflammation, demyelination, astrogliosis, oligodendropathy, and neurodegeneration [1–5]. The aetiopathogenesis of the disease is not fully understood. Infectious, genetic, environmental, and immune factors are known to be involved [6–15]. In the last decades, vitamin D deficiency has been hypothesised as one of the factors that influences the risk of MS [16–20]. It has been suggested that mechanisms dependent on free radicals, imbalance in the antioxidative system, and advanced glycation of proteins may also be involved in the development of this disease [21]. Low molecular weight advanced glycation end-products (AGEs) formed and circulating in the body as a result of the Maillard reaction and those delivered to the body with food (gliotoxins) are removed from the bloodstream by lysosomal degradation via receptors located on the surface of cells. However, most of them are not degraded and accumulate in the body. Their excessive accumulation on cytoplasmic and serum protein, lipid, and nucleic acid molecules leads to oxidative stress and carbonyl stress and the formation of anti-AGE antibodies and cell destructive processes. In addition, AGEs lead to changes in the spatial conformation of proteins and hence the loss of the original properties of these molecules and the disruption of their functions [22–24]. The most common AGEs are Nε-carboxymethyllysine (CML), Nε-carboxyethyllysine (CEL), Nε-lactatolysine, pyrraline, pentosidine, and imidazoles. However, there are many other end-products with unidentified structure and origin [25–29]. CEL is formed by the reaction of methylglyoxal (MGO) with lysine, whereas CML by the reaction of glyoxal (GO) with lysine. Both MGO and GO are major precursors in the formation of AGEs that are formed during glycolysis into which the cell metabolism is switched [30]. Glycation and oxidation of proteins are processes that occur physiologically in the body. However, they can also cause adverse changes in tissues and organs, especially when these processes occur under pathological conditions (e.g. proinflammatory activation). These processes contribute to the formation or intensification of symptoms of many diseases, including neurodegenerative conditions. There are reports of their involvement in the pathogenesis of Alzheimer’s disease [31, 32], Parkinson’s disease [33, 34], and amyotrophic lateral sclerosis [35].
Because the knowledge concerning the potential role of glycated proteins in the pathogenesis of MS is limited [36, 37], the aim of our study was to evaluate the intensity of the protein glycation process in patients with this disease. We hypothesised that MS is accompanied by an intensification of the protein glycation process expressed by elevated serum levels of selected AEGs (i.e. CML, CEL).
Material and methods
The study group consisted of 45 patients with MS (30 women and 15 men; mean age: 38.9 ±9.4 years), who lived in the province of Silesia and were associated with SEZAM, which is the Silesian Association of Multiple Sclerosis (Gliwice, Poland). The control group consisted of 31 healthy volunteers (20 women and 11 men; mean age: 42.4 ±11.3 years). Fasting blood samples were collected from the elbow vein. Serum obtained by centrifugation was stored at –85°C until analysis.
Each MS patient completed the questionnaire related to their history (age, sex, place of residence, onset, duration, and course of the disease, degree of disability, quality of life, and treatment). The medical records were also analysed in detail. Each patient underwent neurological assessment, and the functional status was determined by the Expanded Disability Status Scale (EDSS). The inclusion criteria in the study group were as follows: age ≥ 18 years, MS diagnosis based on McDonald’s criteria (2010), the result of magnetic resonance imaging [38], and informed consent. The exclusion criteria were as follows: neurological comorbidities other than MS (i.e. dementia, previous stroke, neuropathy, cervical radiculopathy, etc.), chronic systemic diseases (i.e. diabetes, advanced heart failure, chronic renal disease, thyroid diseases, autoimmune diseases, etc.), and infectious diseases (especially Lyme disease). The control group consisted of healthy adults with no history of familial neurodegenerative diseases.
The research was approved by the Local Bioethics Committee of the Medical University of Silesia, Katowice. All participants were informed and gave their consent to participate in the study.
The concentrations of selected parameters of advanced protein glycation in serum samples were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits. The carboxymethyllysine levels were measured using the OxiSelect N-(carboxymethyl)lysine (CML) Competitive ELISA Kit, and the carboxyethyllysine levels were measured using the OxiSelect N-(carboxyethyl)lysine (CEL) Competitive ELISA Kit (both from Cell Biolabs Inc., CA) according to the manufacturer’s recommendations. Absorbances were read with the Power Wave XS plate reader (BioTek, Winooski, VT) at 450 nm (reference wavelength – 630 nm), and the results were processed with KC Junior software (BioTek, Winooski, VT). Determinations were performed during one series. The intraassay variation was below 8%. The assay sensitivity was 2.25 ng/ml for CML and 0.1 μg/ml for CEL.
Statistical analysis
The obtained results were presented using the basic parameters of descriptive statistics. The compatibility of distribution of variables with the normal distribution was checked by the Shapiro-Wilk test. Non-parametric Kolmogorov-Smirnov and U Mann-Whitney tests were used for comparisons between the studied groups. The Kruskal-Wallis rank ANOVA test was used to study variability in the MS group. Spearman’s rank test was used for correlations. P < 0.05 was considered statistically significant. The calculations were performed using Statistica for Windows 12.0 (StatSoft, Cracow, Poland).
Results
The study group consisted of 45 patients with MS (67% women, 33% men), and the control group consisted of 31 healthy persons (65% women, 35% men). The gender distribution and age were comparable in both groups (p = 0.86 and p = 0.29, respectively). The MS patients were characterised by different disease duration (from the first symptoms): 0–5 years – 29%; 6–10 years – 33%; 11–15 years – 24%; and 16 and more years – 14%. Relapsing-remitting MS was diagnosed in 73% of patients, and secondary progressive MS in 27% of patients. The assessment of motor function according to the EDSS revealed that 40% of patients had scores ranging from 0 to 1.5 points, 33% of patients from 2 to 4.5 points, and 27% of patients scores ≥ 5 points. The most common symptoms of the disease in the study group included weakness in at least one limb (76%), balance disorders (50%), mood disorders (42%), sensory disturbances (42%), visual impairment (34%), and bladder problems (21%). Twenty patients were treated with disease-modifying drugs (DMD) (interferon β – 13, glatiramer acetate – 2, natalizumab – 2, fingolimod – 3 subjects).
The concentrations of CML and CEL in the serum of MS patients and controls are presented in Table I. No significant difference was found between men and women in both groups (p > 0.05 for each comparison). Serum CML and CEL concentrations did not correlate with the age in the MS group (R = –0.117; p = 0.44 for CML and R = 0.026; p = 0.86 for CEL) or in controls (R = –0.169; p = 0.36 for CML and R = –0.280; p = 0.11 for CEL). The mean serum concentration of CML was significantly higher in MS patients than in healthy subjects. The mean serum concentration of CEL was higher in the study group compared to the controls. However, the difference was not statistically significant (Table II).
Table I
CML and CEL concentrations in the serum of MS patients and healthy controls depending on gender (U Mann-Whitney test)
Table II
CML and CEL concentrations in the serum of MS patients and healthy controls (U Mann-Whitney test)
| Parameter(mean ± SD) | MS patients (n = 45) | Control group (n = 31) | P-value |
|---|---|---|---|
| CML [ng/ml] | 28.86 ±9.40 | 24.06 ±10.33 | 0.031 |
| CEL [ng/ml] | 996.73 ±392.72 | 875.48 ±522.07 | 0.201 |
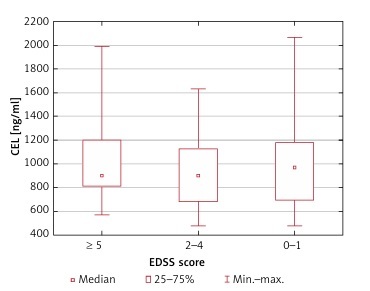
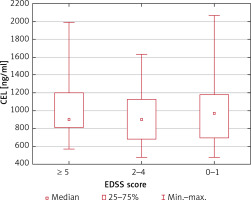
Serum CML and CEL concentrations correlated positively in healthy controls (R = 0.50; p = 0.004) but not in MS patients (R = 0.09; p = 0.54). We found no association between the serum CML and CEL concentrations and the duration of the disease (p = 0.60 and p = 0.75, respectively) and EDSS score (p = 0.78 and p = 0.81, respectively) (Figures 1–4).
Figure 1
CML serum concentrations depending on the duration of MS (no statistically significant differences in the Kruskal-Wallis ANOVA rank test)
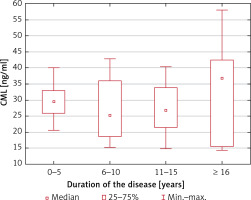
Figure 2
CEL serum concentrations depending on the duration of MS (no statistically significant differences in the Kruskal-Wallis ANOVA rank test)
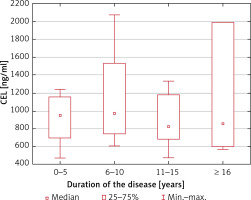
Figure 3
CML serum concentrations depending on the EDSS score (no statistically significant differences in the Kruskal-Wallis ANOVA rank test)
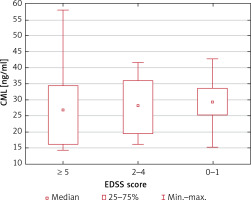
Figure 4
CEL serum concentrations depending on the EDSS score (no statistically significant differences in the Kruskal-Wallis ANOVA rank test)
The mean CML and CEL concentrations were not statistically different between patients treated and untreated with immunomodulatory drugs (p = 0.54 and p = 1.00, respectively). Moreover, the mean CML and CEL concentrations were similar in patients treated with interferon β and those treated with other immunomodulatory drugs (p = 0.61 and p = 0.46, respectively).
Discussion
The results of our study confirm the hypothesis that MS is associated with an intensification of the protein glycation process. We found significantly higher mean serum CML concentrations in MS patients than in healthy controls. The mean serum concentrations of CEL were also higher in MS subjects. However, this difference did not reach the threshold of statistical significance. Contrary to our results, Kalousova et al. [36] did not observe significant differences in total concentrations of AGEs or pentosidine in the cerebrospinal fluid and serum between MS and control groups. Additionally, they did not notice differences in mean concentrations of these parameters between patients with active and stable disease.
On the other hand, our results were partially consistent with the observations of Sternberg et al. [37], who also noticed the association between AGE concentration and MS. However, their results were contrary to ours. The mean CML concentration reported by Sternberg et al. was not significantly different between the MS group and healthy volunteers, while the mean serum CEL concentration was significantly higher in MS patients. Those authors concluded that AGEs, and particularly CEL, might be useful serum MS biomarkers and even suggested the introduction of AGE-inhibitor therapy [37]. Although the difference in CML concentrations between the groups did not reach the statistical significance threshold, the concentration of CML was higher in more than 45% of patients compared to the mean value in the control group. As regards CEL, the concentration of this parameter was higher in 91% of MS patients than the mean values in the controls. Similarly, higher CML and CEL concentrations were found in 69% and 47% of MS patients, respectively, compared to the mean values in the controls.
The observations of Sternberg et al. [37] were different from our results in terms of the association between AGE concentration and gender. Both serum CML and CEL concentrations were significantly higher in healthy men than in healthy women. However, there were no gender differences in MS patients. In our study, no significant differences were found in MS or the control subjects. Sternberg et al. observed higher CML but not CEL concentrations in patients with active MS in comparison to patients with stable disease. In our study group, all MS patients were clinically stable, but serum CML concentrations were significantly higher compared to those in healthy subjects. Sternberg et al. [37] did not report any correlations between serum CML and CEL concentrations either in healthy subjects or in MS patients. In our study, both parameters correlated positively only in healthy controls. In MS patients no correlation was found between serum CML and CEL concentrations, which suggests the existence of additional sources of CML in MS. However, our study revealed that neither CML nor CEL concentrations differed significantly in relation to the degree of disability of patients, which is in line with the observations by Sternberg et al. [37]. Of note, the highest CML concentrations were observed in patients with an EDSS score of more than five points.
The presence of advanced protein glycation products may be associated with inflammation and increased oxidative stress [39]. Higher values of AGEs in MS patients may indicate a higher probability of disease relapse. The increase in plasma AGE concentrations in MS patients can probably be associated with dysfunction of the blood-brain barrier (BBB) [40] or may result from the activation of peripheral immune cells due to chronic inflammation and increased oxidative stress [41]. Although observations concerning the relationship between serum CML and CEL levels and the incidence of MS are not clear, it should be noted that AGEs accumulate in the tissues (e.g. in the brain) and may indicate the ongoing inflammatory process. Wetzels et al. observed that AGE levels were increased in MS lesions due to the inflammatory activation of macrophages and astrocytes [30].
The determination of serum CML and CEL concentrations in the same patients during relapse and remission might provide better information on the utility of AGEs as markers for the risk of subsequent disease relapse [37].
In our study, we did not observe differences in concentrations of the examined parameters between patients treated and untreated with immunomodulatory agents. Sternberg et al. noticed that MS patients receiving immunomodulatory agents had lower mean serum CEL concentrations by 40% compared to untreated subjects, but they were still significantly higher than in the control group. Serum CML concentrations in both treated and untreated MS patients were comparable [37].
Other researchers examined the association between the receptor for advanced glycation end-products (RAGE) and disease-modifying drugs [42]. Rahimi et al. [43] and Asadikaram et al. [44] noticed elevated levels of serum soluble RAGE in patients treated with interferon β. Moreover, Sternberg et al. [45] observed that fingolimod also mediated modulation of the RAGE axis, which apparently contributed to the anti-inflammatory and neuroprotective effects of fingolimod. One-year treatment with this drug increased serum levels of soluble RAGE isoforms (i.e. sRAGE and esRAGE) by 32.4%.
It was demonstrated that AGE levels were also higher in other neurological diseases [31–35]. In addition, AGEs are involved in many pathological processes in the nervous system. They affect myelin proteins, which consequently are susceptible to phagocytosis by macrophages and contribute to demyelination and peripheral neuropathy, e.g. in patients with diabetes [46]. Immunohistochemical studies have also shown an increase in CML concentration in neurons and spinal cord microglia in patients with amyotrophic lateral sclerosis [47]. It has been proven that AGEs affect neurodegeneration (loss of neurons), which not only occurs in the course of diseases commonly considered neurodegenerative, such as Alzheimer’s disease or Parkinson’s disease, but it is also evident in the development of inflammatory and demyelinating diseases of the brain and the spinal cord, such as MS [33, 48, 49]. The pathomechanism of neurodegeneration in MS is not fully understood [50]. Wetzels et al. hypothesised that AGEs could accelerate the occurrence of MS lesions by inducing a proinflammatory activity in microglia and astrocytes. It is also associated with increased RAGE expression. Moreover, AGEs could destroy the BBB function, which leads to the increased infiltration of peripheral immune cells into the central nervous system, leading to neuroinflammation and neurodegeneration [51, 52].
Polymorphism in glutathione transferase-encoding genes was found in MS patients. It leads to a decrease in detoxification and antioxidant potential [53, 54]. However, an increase in the concentration of malonic dialdehyde and a decrease in glutathione peroxidase activity were observed in the cerebrospinal fluid of MS patients [55, 56]. Neurons are particularly sensitive to oxidative disorders due to the increased aerobic metabolism, a high content of unsaturated fatty acids, and relatively low levels of (enzymatic and non-enzymatic) antioxidants. Even short-term oxygen deficiency results in an increase in the concentration of reactive oxygen species and damage to lipids, proteins, and DNA [57]. Hence, a variety of oxidative stress markers have been sought in MS [58].
In conclusion, it should be stressed that the issue of the involvement of the glycation process in the aetiopathogenesis of MS remains open. There is still little research related to this subject, and the results of studies are inconclusive and often contradictory. This study should be another attempt to clarify whether and to what extent post-translational protein processing plays a role in the incidence, course, and severity of MS. It appears that the disease is accompanied by an increase in the glycation processes, especially in metabolic pathways leading to carboxymethyllysine formation. However, it is not possible to decide whether it is the cause or the effect of inflammation. The course of MS also changes some of the relationships between different glycation products, which may suggest additional sources of their formation during the disease process. Further research is warranted to clarify whether and which AGEs could serve as diagnostic and therapeutic biomarkers for MS; it is of great interest, especially in the context of new medicinal implications and the suggestion for the introduction of AGE-inhibitors to the therapy of MS. These inhibitors have also been proposed as promising neuroprotective compounds [59]. It seems that these actions combined with multidisciplinary rehabilitation could improve the quality of life in patients with MS [60].
Multiple sclerosis is accompanied by an intensification of protein glycation process, especially within the pathways leading to the formation of carboxymethyllysine. The duration of the disease and the degree of disability seem not to affect the progression of the glycation process. However, the disease process associated with MS may affect the relationship between CML and CEL concentrations.
The small size of patient groups and subgroups (i.e. treated and untreated with DMD) could influence statistical differences. We examined only clinically stable patients. However, it would be interesting to compare serum CEL and CML levels during relapse and remission in the same patients. Therefore, further studies are warranted in this respect.