Multiple myeloma (MM) is a malignant clonal disorder of plasma cells, for which no cure has been found. Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative tumor first described in 1920 [1]. The World Health Organization (WHO) officially named and classified CNL in 2001 and revised its diagnostic criteria in 2008 [2–4]. The coexistence of MM and CNL is exceptionally rare, complicating the clinical diagnosis and frequently leading to misdiagnosis or underdiagnosis. In our hematology department, we managed a case of MM and CNL in a patient with CSF3R (Gln776X) and ASXL1 mutations. Given the scarcity of reported cases, this study aims to discuss the diagnosis, treatment, and pathogenesis of this rare condition, combining our findings with relevant literature.
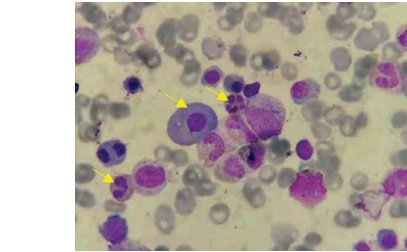
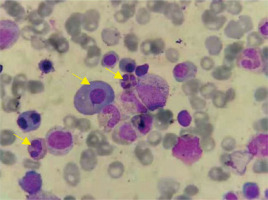
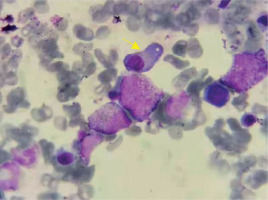
On April 23, 2019, an 86-year-old male patient diagnosed with MM and CNL was admitted. The patient presented with a “pulmonary infection accompanied by pleural effusion”. During anti-infective therapy, there was a persistent abnormal elevation in white blood cell count. Hematologic examination revealed a white blood cell count of 67.90 × 109 /l, predominantly mature neutrophils (64.31 × 109 /l) (Figures 1, 2). Biochemical analysis indicated an elevated globulin level of 41.7 g/l. Immunoglobulin analysis showed an IgA level of 3640.0 mg/dl. Serum immunofixation electrophoresis revealed positive results for IgA and λ light chain. Free light chain assay indicated λ at 1790 mg/l in blood and 9610 mg/l in urine. Peripheral blood cell classification showed 92% segmented neutrophils and 8% mature lymphocytes. The neutrophil alkaline phosphatase score was 226. Bone marrow cytology revealed marked hyperplasia with a predominance of granulopoiesis (78.8%), including mature neutrophils, myelocytes, metamyelocytes, and segmented neutrophils. Plasma cells constituted 12%, with 3.2% being immature and pro-plasma cells, and bi-nucleated and multi-nucleated plasma cells were observed. Flow cytometry demonstrated approximately 0.75% monoclonal plasma cells. The MM prognosis fluorescence in situ hybridization (FISH) panel showed negativity for P53, RB1, 1q21, D13S319, and IGH. A comprehensive myeloproliferative neoplasm (MPN) gene panel revealed negativity for BCR/ABL, JAK2, CALR, and MPL. AML/MDS/MPN targeted gene sequencing identified positive mutations in CSF3R (Gln776X) and ASXL1. Chromosomal karyotyping revealed a normal male karyotype of 46, XY. PET-CT imaging demonstrated diffuse abnormal elevation in FDG metabolism along the axial skeleton. The diagnosis of MM and CNL (CSF3R (Gln776X) with ASXL1 mutation) was confirmed. The procedures in this study adhered to the 2013 revised Declaration of Helsinki. Informed consent was obtained from the patient’s family, and they signed a clinical research consent form.
During hospitalization, the patient underwent various laboratory and auxiliary examinations. These included CT, CBC, peripheral blood cell morphology, NAP score, bone marrow smear, immunoglobulin measurement, serum protein electrophoresis, immunofixation electrophoresis, blood and urine free light chain assay, coagulation function and biochemical parameters, bone marrow flow cytometry, MM prognosis FISH panel, MPN gene panel, AML/MDS/MPN mutational gene sequencing, chromosomal karyotyping, and PET-CT. These examinations provided a comprehensive profile of the patient’s health status, aiding in diagnostic and prognostic evaluation.
MM diagnosis requires at least one of the following criteria to be met in addition to the initial two: 1) clonal plasma cells ≥ 10% in bone marrow or tissue biopsy confirmation of a plasma cell tumor; 2) presence of M protein in serum or urine; 3) abnormal target organ function or bone marrow tumor indicators: a) target organ damage (CRAB symptoms); b) no target organ damage but presence of SLiM symptoms. Treatment for MM typically involves proteasome inhibitors, immunomodulatory agents, and dexamethasone, followed by autologous hematopoietic stem cell transplantation (ASCT) post-induction remission [5].
CNL diagnostic criteria, revised by the WHO in 2016 [6], include: 1) peripheral blood white cell count ≥ 25 × 109 /l, with neutrophilic bands and segmented nuclei ≥ 80%, and rare promyelocytes, myelocytes, and metamyelocytes; 2) increased bone marrow activity with elevated neutrophil count and normal maturation, less than 5% primitive granulocytes; 3) exclusion of BCR/ABL-positive chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), or chronic idiopathic myelofibrosis (CIMF); 4) absence of PDGFRα, PDGFRβ, FGFR1, or PCM1-JAK2 rearrangements; 5) presence of CSF3R T618I or other activating CSF3R mutations. Treatment includes allo-HSCT, cytoreductive therapy, and targeted drugs such as ruxolitinib, though there is no standardized treatment, and prognosis remains poor, with a median survival of approximately 2 years [7].
The patient presented with a “pulmonary infection with pleural effusion”. CT scans indicated bilateral lung infections with pleural effusion. The patient did not have fever, bone pain, or mucocutaneous bleeding. Treatment included antimicrobial therapy and closed thoracic drainage for pleural effusion. The patient had a history of diabetes for over 20 years, managed with insulin, and irregular blood glucose monitoring. There was no significant family history.
Initial blood examination showed a white blood cell count (WBC) of 67.90 × 109 /l, with a neutrophil count of 64.31 × 109 /l and a neutrophil percentage of 95.4%. Other values included hemoglobin (HGB) at 119 g/l and platelet count of 188 × 109 /l. Biochemical analysis showed uric acid at 737.0 μmol/l, creatinine at 98.0 μmol/l, urea at 10.10 mmol/l, glycosylated serum protein at 5.5 mmol/l, β2-microglobulin at 8.84 mg/l, globulin at 41.7 g/l, and albumin at 29.1 g/l. Immunoglobulin analysis indicated an IgA level of 3640.0 mg/dl (normal: 82–453 mg/dl). Serum immunofixation electrophoresis was positive for both IgA and λ light chains. Free light chain assay revealed κ at 18.2 mg/l (normal: 6.7–22.4 mg/l), λ at 1790 mg/l (normal: 8.3–27.0 mg/l), and a κ/λ ratio of 0.010 (normal: 0.31–1.56). Urine free light chain analysis showed κ at 140 mg/l (normal: 0–25.8 mg/l), λ at 9610 mg/l (normal: 0–11.3 mg/l), and a κ/λ ratio of 0.015. Peripheral blood cell classification indicated 92% segmented neutrophils and 8% mature lymphocytes. The neutrophil alkaline phosphatase (NAP) score was 226. Bone marrow cytology showed significant myeloid cell proliferation (78.8%), mainly mature neutrophils, myelocytes, metamyelocytes, and segmented neutrophils. The percentage of red blood cells was 2%, mature lymphocytes 7.2%, and plasma cells 12%, including 3.2% immature and pro-plasma cells, with bi-nucleated and multi-nucleated plasma cells observed. Flow cytometry indicated approximately 0.75% monoclonal plasma cells, positive for CD38, CD138, cλ, and negative for CD56, CD19, and cκ. FISH panel was negative for P53, RB1, 1q21, D13S319, and IGH. MPN gene panel screening was negative for BCR/ABL, JAK2, CALR, and MPL. AML/MDS/MPN mutational gene sequencing showed positive mutations in CSF3R (Gln776X) and ASXL1. Chromosomal karyotyping was normal (46, XY). PET-CT imaging showed diffuse abnormal elevation in FDG metabolism along the axial skeleton.
Based on the clinical presentation and laboratory findings, the patient was diagnosed with: 1) multiple myeloma (smoldering type, IgA-λ light chain subtype) and 2) chronic neutrophilic leukemia (CNL). The patient’s family declined treatment. Follow-up revealed that the patient survived for 4 months after discharge.
Approximately 20% of CNL patients have underlying tumors, often MM [8–11]. Clinical manifestations include hepatosplenomegaly and mucocutaneous or gastrointestinal bleeding in 25–30% of patients [8–11]. Many diagnosed patients lack specific clinical symptoms, leading to frequent underdiagnosis. The coexistence of MM and CNL is even rarer, complicating diagnosis and often resulting in misdiagnosis. Including CSF3R mutations in diagnostic criteria has provided specific markers, reducing misdiagnosis rates.
In the present case, the patient exhibited persistent leukocytosis after recovering from a pulmonary infection. Further tests, including MPN comprehensive gene screening and myeloid tumor second-generation sequencing, revealed CSF3R Gln776X and ASXL1 mutations. According to the latest WHO criteria, this confirmed diagnoses of smoldering multiple myeloma (SMM) and CNL. Apart from elevated white blood cell counts, other cell lines were normal at diagnosis. Clinical presentations varied, with some patients asymptomatic at diagnosis, and the time from onset to final diagnosis ranged from several months to years. Therefore, CSF3R mutation testing is crucial in diagnosing MM patients with persistent leukocytosis, especially when antimicrobial therapy is ineffective. CSF3R, a transmembrane receptor binding with G-CSF, plays a crucial role in granulocyte proliferation and differentiation [12]. The discovery of CSF3R mutations has advanced CNL research, with an 80–100% positivity rate in CNL cases [8, 13–15].
CNL patients typically have a median survival of around 2 years, with poor prognosis [11, 13, 16]. Diagnosed WBC levels ≥ 50 × 109 /l and ASXL1 mutations are adverse prognostic factors [9, 14]. Our patient, with CSF3R Gln776X and ASXL1 mutations, had a WBC count ≥ 50 × 109 /l and died after 4 months without treatment, suggesting these as potential adverse factors for MM combined with CNL.
Given the rarity of MM with CNL, no standardized treatment exists. MM is primarily treated with proteasome inhibitors, immunomodulatory agents, and dexamethasone, followed by autologous stem cell transplantation (ASCT) [5]. Traditional treatments such as hydroxyurea and interferon are effective for CNL but limited during progression. The age characteristics of CNL limit allo-HSCT’s clinical application. Ruxolitinib has shown clinical improvement in some cases, but its efficacy remains inconsistent [8, 17–20]. For MM with CNL, treatment with a myeloma regimen achieved disease control, suggesting its effectiveness for CNL [8, 21]. Future research should focus on the molecular mechanisms and clinical applications of JAK inhibitors, SRC kinase inhibitors, and demethylating agents in treating CNL.
In conclusion, MM and CNL patients with CSF3R Q776X and ASXL1 mutations are rare. Treatment approaches for MM may manage this disease type effectively, but the effectiveness of JAK inhibitors, SRC kinase inhibitors, and demethylating agents warrants further research and confirmation.