Uterine corpus endometrial carcinoma (UCEC) is a type of cancer that originates in the uterine endothelium [1]. It is considered as the fifth leading cause of cancer-related death among women, with a majority of cases being diagnosed at an advanced stage due to the lack of noticeable early symptoms. There are two main types of UCEC: hormone dependent and hormone non-dependent [2, 3]. UCEC is primarily a disease of postmenopausal women, with the majority of cases occurring in women aged 50 and older. Key risk factors include obesity, unopposed estrogen therapy (estrogen without progesterone), tamoxifen therapy for breast cancer, a history of polycystic ovary syndrome (PCOS), nulliparity (never having given birth), late menopause, and a family history of endometrial or other related cancers [4]. In some cases, a biopsy may be necessary to confirm the diagnosis. Treatment typically consists of surgery to remove the tumor and chemotherapy to kill any remaining cancer cells. In recent years, targeted therapies and immunotherapies have also become part of the treatment landscape for some patients with endometrial cancer [5].
The role of inflammation in the pathogenesis of UCEC is pivotal, yet the specific mechanisms by which it induces UCEC are not fully understood. Additionally, the peripheral profiles of inflammation and metabolites in UCEC patients have not been comprehensively characterized. Inflammation plays a key role in the development of endometrial carcinoma. Mutations in PTEN are common in endometrial cancers, particularly those classified as type I, which are estrogen-dependent and often have a better prognosis [6]. TP53 is a tumor suppressor that helps regulate cell division. Mutations in TP53 are more commonly associated with type II endometrial cancers, which are not estrogen-dependent and tend to have a worse prognosis [2, 7].
Mendelian randomization (MR) analysis, a method widely applied in drug target development and drug repurposing, employs genetic variants associated with risk factors as instrumental variables [8–10]. This approach simulates the random inheritance of genetic variants from parents to offspring, thereby circumventing issues related to confounding factors and reverse causality. However, there have been few MR studies that integrate genome-wide association studies (GWAS) to examine the impact of immune cell and metabolite changes on ovary cancer. In this study, we aimed to identify immune cell profiles and metabolic alterations as potential biomarkers for ovary cancer, contributing to a deeper understanding of the disease’s pathophysiology and potentially leading to the development of novel therapeutic strategies.
Methods
Medical ethics
For this study, we accessed GWAS data from the public dataset available on the IEU website and GEO dataset data. The study was approved by the local hospital ethical committee, as all data used here are from a public dataset.
Study design and data sources for Mendelian randomization
Drawing on previous research, we employed a two-sample MR approach to estimate the causal effects of key genes on trigeminal neuralgia, utilizing 1400 metabolites and 91 inflammatory factors as instrumental variables (IVs). Single nucleotide polymorphisms (SNPs) serving as IVs for each trait were derived from published GWAS data and clustered to ensure genetic independence (linkage disequilibrium r2 < 0.001; clumping distance of 1000 kb). An F-statistic exceeding ten was deemed sufficiently robust to predict the exposure of interest [8–10]. We utilized datasets from individuals of European ancestry to minimize selection bias and enhance the reliability of our analysis.
Outcome data
We sourced GWAS summary data on trigeminal neuralgia from contributing studies, encompassing 331,976 participants of European ancestry, including 1599 cases and 330,377 controls (G6_TRINEU). The coefficients for each SNP were converted into log odds ratios (OR).
Statistical analysis for MR
Employing the two-sample MR method, we assessed the causal impact of each metabolite or immune cell expression quantitative trait loci (eQTL) on the outcome, presenting results as OR and 95% confidence intervals (CIs). The harmonization of SNP exposure and outcomes has been detailed in previous literature [8–10]. We applied five MR techniques (random-effect IVW, weighted median, MR-Egger, simple mode, and weighted mode) to explore the potential link between key genes and trigeminal neuralgia. The IVW regression presumes that all genetic variants are valid instruments, assuming no pleiotropy [8–10]. The weighted median approach evaluates the robustness of consistent estimation when more than 50% of the IVs are valid [8–10]. MR-Egger analysis, using weighted linear regression between SNP exposure and outcome, was performed to detect potential horizontal pleiotropy through the intercept. Heterogeneity was assessed using scatter plots of causal estimates from multiple genetic variants [8–10].
Sensitivity analysis
In our sensitivity analyses, we compared causal estimates from various MR methods, including MR-Egger, penalized weighted median, simple mode, IVW, and weighted mode, to reinforce the robustness of our findings. Forest plots were utilized to evaluate the causal effects of individual SNPs and to compare these against the causal estimates derived from IVW and MR-Egger approaches using all enrolled SNPs.
Validation with MR-IVW
For MR-derived causal estimates to be valid, three assumptions must be satisfied: (1) the genetic variants are strongly associated with the exposure, (2) the variants are not associated with any potential confounders of the exposure-outcome association, and (3) the variants do not influence the outcome independently of the exposure. To ensure that instruments influence the outcome solely through exposure, we excluded SNPs with strong associations with the outcome. The effects of SNPs on exposure and outcome were harmonized to align the β values with the same alleles.
Results
We first investigated the association between circulating inflammatory factors and the onset of UCEC. We found that FGF23 has a positive relationship with UCEC, while FGF5, CD5, IL-20, CSF1 and uPA have a negative relationship with UCEC (Supplementary Figure S1). We further validated this with TCGA data and found that FGF5, CSF1 and IL-20 are lowly expressed in UCEC tissue compared to normal UCEC tissue with GTEx (Supplementary Figure S2). In addition, we found that only CSF1 is a protective factor in UCEC as its expression is related to the tumor stage of UCEC patients (Supplementary Figure S2).
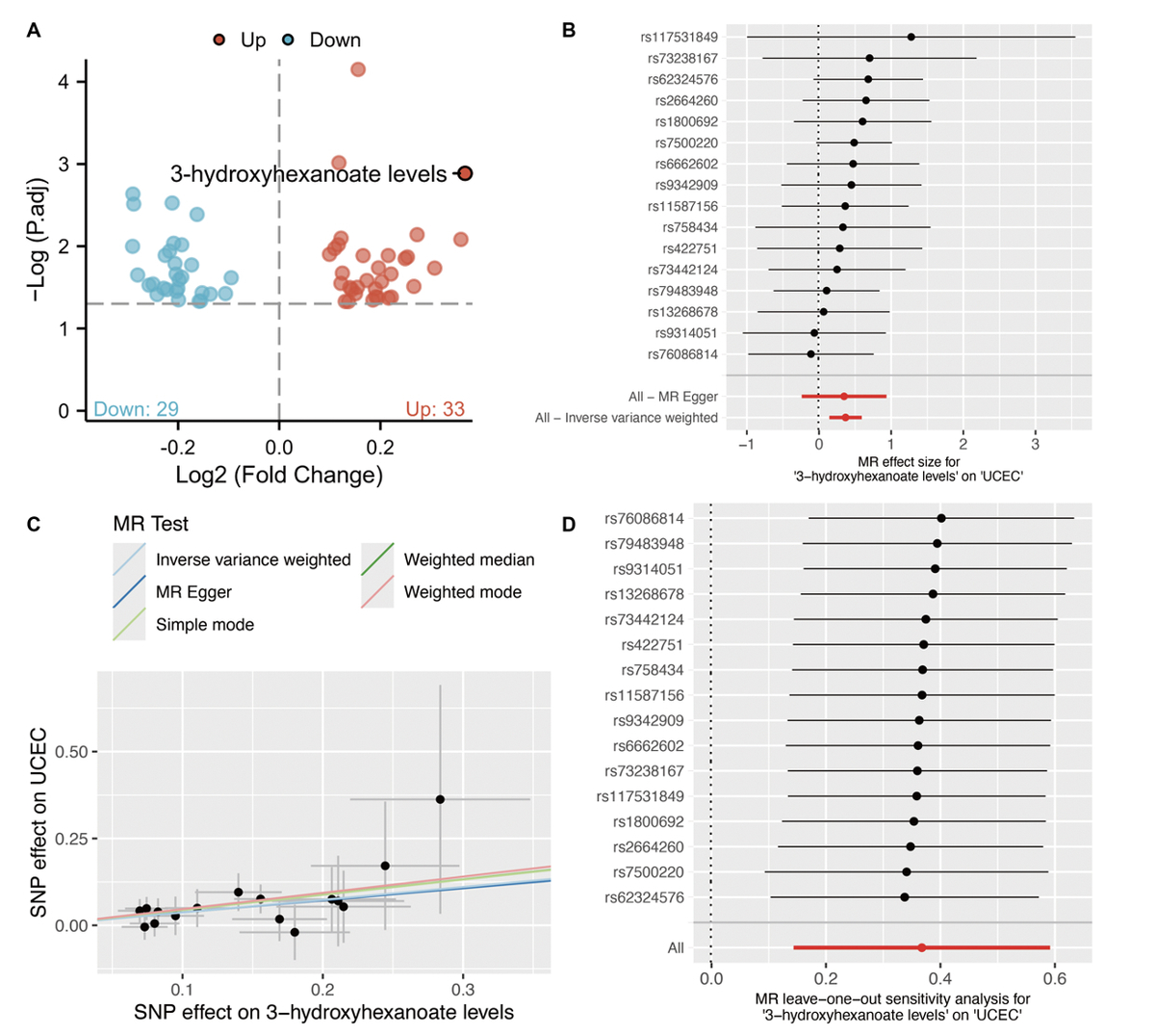
We then investigated the association between circulating metabolites and the onset of UCEC. We found that 33 metabolites are positively associated with UCEC and 29 metabolites are negatively associated with UCEC (Figure 1). Among them, 3-hydroxyhexanoate level is negatively linked with CSF1 (OR = 0.911, p = 0.015, Figure 2).
Figure 1
MR analysis for circulating metabolites and UCEC. A – Volcano plot shows the relationship between circulating metabolites and UCEC. B – Forest plot of individual and combined SNP MR-estimated effect sizes. C – Scatter plot of SNP effects on 3-hydroxyhexanoate versus UCEC, with the slope of each line corresponding to the estimated MR effect per method. D – Leave-one-out analysis shows the MR leave-one-out sensitivity analysis for 3-hydroxyhexanoate on UCEC
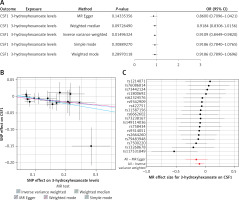
Figure 2
MR analysis between 3-hydroxyhexanoate level and CSF1 with OR = 0.9186 and p-value = 0.0150 (IVW result). A – MR results between 3-hydroxyhexanoate level and CSF1; B – Scatter plot of the MR results between 3-hydroxyhexanoate level and CSF1. C – Forest plot of the MR results between 3-hydroxyhexanoate level and CSF1
Discussion
The role of inflammation in endometrial cancer is complex and multifaceted, involving interactions between various cytokines, growth factors, and immune cells. Understanding these interactions can provide insight into potential therapeutic targets for reducing inflammation and inhibiting tumor progression in UCEC. Anti-inflammatory agents and lifestyle changes that reduce systemic inflammation may be beneficial in the prevention and management of endometrial cancer [11].
CSF1 has been implicated in the migration of tumor cells or endothelial cells. Additionally, the reduction in CSF1-dependent tumor-associated macrophages (TAMs) resulted in increased CD8+ T cell attack on tumors, but its effect on tumor growth was limited by a compensatory increase in Foxp3+ Treg cells [12].
Potential therapeutic target: Given the dysregulation of CSF1 in tumor tissues, it has been suggested that analyzing and measuring CSF1 could predict tumor progression. Furthermore, therapies targeting the CSF1 axis are being explored as a potential approach to overcome tumor progression [13].
Interaction with other chemokines: CSF1 is part of the chemokine network that plays a key and complex role in tumor progression. For instance, T-cell induced CSF1 promotes melanoma resistance to PD1 blockade [14]. Meanwhile, CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy [15].
This study employed MR analysis to investigate the causal relationship between 3-hydroxyhexanoate level and UCEC, with a particular focus on the mediating role of CSF1. Our findings provide novel insights into the pathophysiological mechanisms underlying UCEC and highlight potential biomarkers and therapeutic targets for this endometrial disorder.
The positive association between 3-hydroxyhexanoate level and UCEC, as revealed by our MR analysis, suggests that this metabolite may play a detrimental role in the disease’s pathogenesis [2, 16, 17]. 3-Hydroxyhexanoate is a mediating metabolite during fatty acid β-oxidation, which has been studied for its potential effects on cancer cells and cancer treatment-related symptoms [18]. Cancer cells have altered metabolism and can be particularly reliant on certain nutrients for energy. 3-Hydroxyhexanoate’s role in fatty acid transport into mitochondria for β-oxidation might affect cancer cell metabolism, potentially influencing cancer growth and progression. There has been some research into the levels of 3-hydroxyhexanoate in cancer patients’ plasma, including breast cancer patients [19], to determine whether they could serve as potential biomarkers for diagnosis or prognosis. However, more research is needed to establish any clear link.
Furthermore, the identification of CSF1 as a mediator in the relationship between 3-hydroxyhexanoate and UCEC is a significant finding. We have also validated the lower expression of CSF1 in UCEC tissue from the TCGA dataset.
The results of this study contribute to the growing body of evidence implicating metabolic dysregulation and proinflammation in UCEC. Understanding the interplay between metabolites and inflammation in the disease’s etiology could lead to the development of more targeted and effective treatments for ovary cancer. Moreover, the use of MR analysis in this study helps to address some of the limitations associated with observational studies, such as confounding and reverse causality. By utilizing genetic variants as instrumental variables, our study provides stronger evidence for a causal relationship between 3-hydroxyhexanoate, CSF1 and UCEC. Therefore, both inflammatory and metabolic markers could become biological markers for UCEC during diagnosis and therapy.
While our study offers valuable insights, it is not without limitations. The cross-sectional nature of the GWAS data and the reliance on MR analysis mean that further longitudinal studies and experimental validations are needed to confirm the causal relationships identified. Additionally, the specific mechanisms by which 3-hydroxyhexanoate and CSF1 interact in the context of UCEC require further investigation.
Future research should also explore the potential therapeutic implications of targeting 3-hydroxyhexanoate metabolism or CSF1 in ovary cancer. This could involve clinical trials assessing the efficacy of combined interventions aimed at modulating these pathways in patients with ovary cancer.
In conclusion, our MR study identified a potential causal link between 3-3-hydroxyhexanoate, CSF1, and UCEC. These findings not only enhance our understanding of the disease’s pathophysiology but also point towards possible new avenues for therapeutic intervention. Further research is needed to fully elucidate the mechanisms involved and to translate these insights into improved treatments for patients with UCEC.