Lung cancer is a disease that seriously endangers human health [1, 2]. The overall 5-year survival rate of patients with newly diagnosed lung cancer is still only about 15%, and the majority of instances of lung cancer are in the advanced stage despite advances in screening, diagnosis, and multimodal treatment modalities. In recent years, immunotherapy has achieved many major breakthroughs in the cancer field. With the continuous deepening of tumor immunity and immune tolerance, lung cancer immunotherapy has become a popular topic for current cancer research.
According to tumor immunology, the body’s immunological health has a direct impact on the occurrence and progression of cancer. It has been reported that adenosine, which plays a key immunosuppressive role in the tumor microenvironment, can both stimulate the development of regulatory T lymphocytes and simultaneously repress other immune cells, thereby allowing the escape of tumor cells. The CD39 molecule, as the initiating molecule in adenosine metabolism, has a significant impact on adenosine production. NTPDase, also known as ecto-nucleoside triphosphate diphosphohydrolase (CD39), controls the balance of the immunological response by hydrolyzing adenosine triphosphate (ATP) and adenosine diphosphate (ADP). Immunotherapy, which modifies the immunological system of patients, has assumed an increasingly significant role in the treatment of tumors in recent years. Programmed cell death protein 1 (PD-1) checkpoint inhibitors are one of several therapies that have been authorized for the treatment of numerous malignancies, although they are only successful in some people [3–5]. When it comes to the appropriate activation of CD8-positive T lymphocytes, CD28 plays a crucial function as a crucial costimulatory molecule that is essential for T cell activation. According to recent research, senescent CD8-positive T lymphocytes that behave immunosuppressively in malignancy are characterized by downregulation of CD28.
In this study we wanted to find out whether CD39 has a regulatory effect on immunity as an important molecule in the adenosine metabolic pathway, and whether CD39 is associated with PD-1 and CD28. We first detected the CD39 expression on CD8-positive T lymphocytes and combined with the clinical information of lung adenocarcinoma patients to explore the role of CD39. Then, correlation studies were carried out to analyze the relationship of CD39 with antitumor cytokines, PD-1 and CD28. This study provides evidence to support further investigation into the mechanisms through which CD39 negatively affects the immune function of lung adenocarcinoma patients.
Methods
Patients and study design
Peripheral blood samples were obtained from 59 healthy people (age distribution of 32–82 years) and 203 lung adenocarcinoma cancer patients (age distribution of 41–84 years) who were first admitted to Tianjin Cancer Hospital Airport Hospital after receiving written informed consent. The Ethics Committee of Tianjin Cancer Hospital Airport Hospital gave its approval to the procedure. Prior to blood collection, none of the patients received surgery, radiotherapy, chemotherapy, or other medical intervention.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Cancer Hospital Airport Hospital in September 2022. The approval number is JSK-2022-0062.
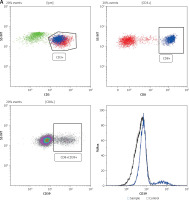
The cells were treated with adequately diluted antibodies after FcR had been blocked, and then phosphate buffered saline (PBS) was applied to wash the cells. The antibodies used for surface staining included anti-human CD45-PC7/CD8-ECD/CD3-PC5 (Beckman Coulter), anti-human CD39-PE (BioLegend), anti-human CD28-FITC (BioLegend) and anti-human CD279-APC (BioLegend). Acquisition was performed using a Navios flow cytometer (Beckman Coulter). Data analysis was conducted using Kaluza Software (Beckman Coulter). The flow cytometry analysis process is shown in Figure 1 A. The serum from the patients was first incubated with appropriate cytokine capture microspheres (Wellgrow) for an hour, washed with washing buffer, then incubated with the indicator antibody (Wellgrow) for an additional hour before being again washed with washing buffer. Acquisition was performed using a DxFlex flow cytometer (Beckman Coulter). Data analysis was conducted using WELLCKAS Software (Wellgrow).
Figure 1
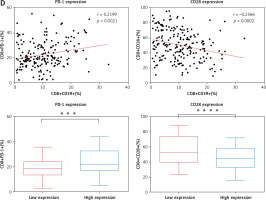
CD39 expression on CD8-positive T lymphocytes is correlated with clinical characteristics, cytokines, and the expression of PD-1 and CD28. A – Representative flow cytometry gating strategies for T lymphocytes and CD39 expression in the peripheral blood of lung adenocarcinoma patients B – Correlation between the expression of CD39 on CD8-positive T lymphocytes in the peripheral blood of lung adenocarcinoma patients and clinical characteristics, C – The relationship between CD39 expression on CD8-positive T lymphocytes and the secretion of two antitumor cytokines, IFN-γ and TNF-α, in patients with lung adenocarcinoma D – The relationship between CD39 expression and PD-1 and CD28 expression on CD8-positive T lymphocytes in patients with lung adenocarcinoma
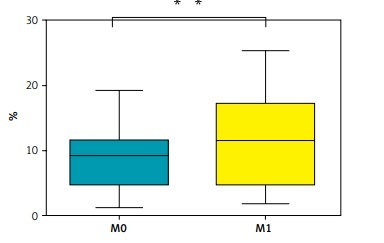
N0 – lung adenocarcinoma patients without lymphatic invasion, N1N2N3 – lung adenocarcinoma patients with lymphatic invasion, M0 – lung adenocarcinoma patients without distant metastasis, M1 – lung adenocarcinoma patients with distant metastasis. The p-value shown is obtained from the comparison between the indicated groups by the nonparametric t test. *P < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.

Statistical analysis
SPSS Statistics 21 (IBM Corporation, NY, USA) was used for all statistical analyses. As the Kolmogorov-Smirnov test showed that the numerical data had a normal distribution, the mean and standard deviation were used to represent the data. The independent sample t-test was used to compare numerical data. P < 0.05 was considered statistically significant.
Results
The relationship between CD39 expression on CD8-positive T lymphocytes in peripheral blood and clinical information in patients with lung adenocarcinoma
This study detected the surface expression of CD39 on CD8-positive T lymphocytes in the peripheral blood from patients with lung adenocarcinoma and conducted a correlation analysis with clinical information. The findings revealed that in patients with lung adenocarcinoma, the expression of CD39 on CD8-positive T lymphocytes was related to T stage, lymph node metastasis, and distant metastasis, as illustrated in Figure 1 B and Table I. Therefore, the results show that lung adenocarcinoma patients who are in stage T4 with N4 and distant metastasis have more CD8+CD39+ T cells than other lung adenocarcinoma patients.
Table I
Correlation analysis between the percentage of CD8+CD39+ T cells in the peripheral blood of patients with lung adenocarcinoma and clinical characteristics
CD8+CD39+ T cells are weakened CD8+ T subsets secreting lower levels of antitumor cytokines
We employed flow cytometry to measure the level of cytokine production in the peripheral blood of patients with lung adenocarcinoma in order to examine the potential role of CD8+CD39+ T lymphocytes. This study detected two cytokines (IFN-γ and TNF-α) related to antitumor cytotoxicity. We first analyzed whether there was a correlation between the percentage of CD8+CD39+ T lymphocytes in CD8-positive T lymphocytes and the secretion level of the two antitumor cytokines. The findings revealed a correlation between the cytokines IFN-γ and TNF-α and the percentage of CD8+CD39+ T lymphocytes (Figure 1 C). There was a negative correlation between the secretion of two antitumor cytokines and CD39 expression on CD8-positive T lymphocytes. In accordance with the median proportion of CD8+CD39+ T lymphocytes in CD8-positive T lymphocytes, we separated all patients into two groups: one with high CD39 expression and the other with low expression. By analyzing the secretion level of these two antitumor cytokines between the two groups, the results show that the CD39 low expression group can secrete more IFN-γ and TNF-α (Figure 1 C). Together, these findings indicated that CD8+CD39+ T lymphocytes may weaken CD8+ T subsets by secreting lower levels of antitumor cytokines, which affects the progression of lung adenocarcinoma.
The relationship between CD39 expression and the expression of two important molecules (PD-1 and CD28) on CD8+ T lymphocytes in lung adenocarcinoma patients
The association between CD39 expression and PD-1 and CD28 expression on CD8-positive T lymphocytes in lung adenocarcinoma patients was the subject of our next investigation. PD-1 and CD28 were substantially correlated with CD39 expression on CD8-positive T lymphocytes (Figure 1 D). PD-1 expression was positively correlated with CD39 expression, while the CD28 expression was negatively correlated with CD39 expression. All patients were classified into two groups based on the median (%) expression of CD8+CD39+ T lymphocytes in CD8-positive T lymphocytes. Figure 1 D illustrates the dramatically varied expression of these two important molecules between the groups with low and high expression. As a result, these two important molecules differ significantly depending on different CD39 expression groups, and the CD39 high expression group can express more PD-1 and lower CD28.
Discussion
CD39, as the main rate-limiting enzyme in the adenosine metabolism pathway, can hydrolyze ATP/ADP, thereby playing an important role in the microenvironment of the tumor [6, 7]. CD39 has been widely studied as an immunosuppressive molecule in the progression and development of various cancers [8–10]. But as the research advances, more and more data show that CD39, a protein found on the surface of many cells, serves a number of biological purposes [11, 12]. CD39 expression can not only induce regulatory T-cell conversion and generation, but also prevent the transformation of Th17 cells and inhibit the production of IL-17, thus reducing the development of autoimmune and chronic inflammatory diseases [13, 14].
CD39 expression on CD8-positive T lymphocytes is significantly higher in patients who are in stage T4 with N4 and distant metastasis than in other lung adenocarcinoma patients. Patients with advanced lung adenocarcinoma had considerably higher levels of CD39 expression than those with early medium-stage lung adenocarcinoma, suggesting that CD39 expression has a negative correlation with the absolute number of CD8-positive T lymphocytes. CD39 has the characteristics of negative immune regulation, which can cause the exhaustion of CD8-positive T lymphocytes by certain mechanisms, leading to a decrease in the number of CD8-positive T lymphocytes and ultimately resulting in tumor progression and metastasis. In conjunction with the existing research, this study analyzed the expression of the negative regulatory CD39 molecule in patients with lung adenocarcinoma. The study hypothesized and demonstrated a negative correlation between CD39 expression and CD8-positive T lymphocytes’ function.
Combined with the discovery of this study, we can suppose that CD39 expressed on the surface of CD8-positive T lymphocytes, as a negative regulatory molecule, can cause the exhaustion of CD8-positive T lymphocytes (secreting lower levels of the antitumor cytokines IFN-γ and TNF-α) and a decrease in the number of CD8-positive T lymphocytes by certain intracellular signaling pathways. In addition, CD39 can further change the expression of some immune checkpoints in T lymphocytes, such as PD-1 and the costimulatory molecule CD28, exerting negative immune regulation. According to the report, suppressor CD8+CD28- T lymphocytes can be detected in the peripheral blood of 73% of cancer patients [15]. So CD39 can reduce the CD28 expression and induce the production of suppressor CD8+CD28– T lymphocytes. Our study suggests that CD39, the key molecule in adenosine metabolic pathways, may also have the characteristics of negative immune regulation, which can cause the exhaustion of CD8-positive T lymphocytes by certain mechanisms, or lead to a decrease in the number of CD8-positive T lymphocytes, ultimately resulting in tumor progression and metastasis.
In conclusion, we hope that by detecting the lymphocyte subsets of the peripheral blood in tumor patients, it is possible to provide more valuable information for the clinic, and a clinical treatment strategy can be developed according to the immune function state of each patient. Finally, patients can obtain optimized benefits from tests. In addition, this investigation found that CD39 expression was negatively correlated with CD8-positive T lymphocytes’ function, providing a basis for subsequent clarification of CD39 as a negative regulator.