Introduction
Deep venous thrombosis (DVT) stands as a prominent cause of global disability, significantly contributing to the overall burden of disease. Unraveling potential causal factors for DVT and understanding their impact direction could aid in formulating effective prevention strategies during clinical interventions. Beyond established risk factors like pregnancy, stressors, and certain sociodemographic elements [1, 2], the interplay between anticoagulation and DVT has gained considerable attention [2, 3].
While both body mass index (BMI) and obesity have been associated with DVT, lipid profiles lack a genetic link to DVT [4]. Observational studies indicate a reduced anticoagulation factor in individuals with DVT [5], yet the causal relationship with antithrombin and DVT remains contentious. This hypothesis gains support from the negative correlation between coagulation factors and DVT [6, 7]. Some studies propose that decreased anticoagulation proteins correlate with increased DVT risk, as protein C facilitates anticoagulation, resolving thrombosis and improving outcomes [8, 9]. However, data on the association between plasma protein C, protein S, antithrombin-III (AT-III), and DVT onset have yielded inconclusive and controversial results, given the connection between increased activated protein C (APC) resistance and recurrent spontaneous abortion [10]. Other observational studies and one randomized controlled trial have shown increased mortality risk when relative protein C level is increased via giving fresh frozen plasma [11]. Activated protein C can interact with thrombin, which plays a role in suppressing coagulation by deactivating clotting V and VIII factors [12]. Vitamin status is also associated with the human health situation [13, 14].
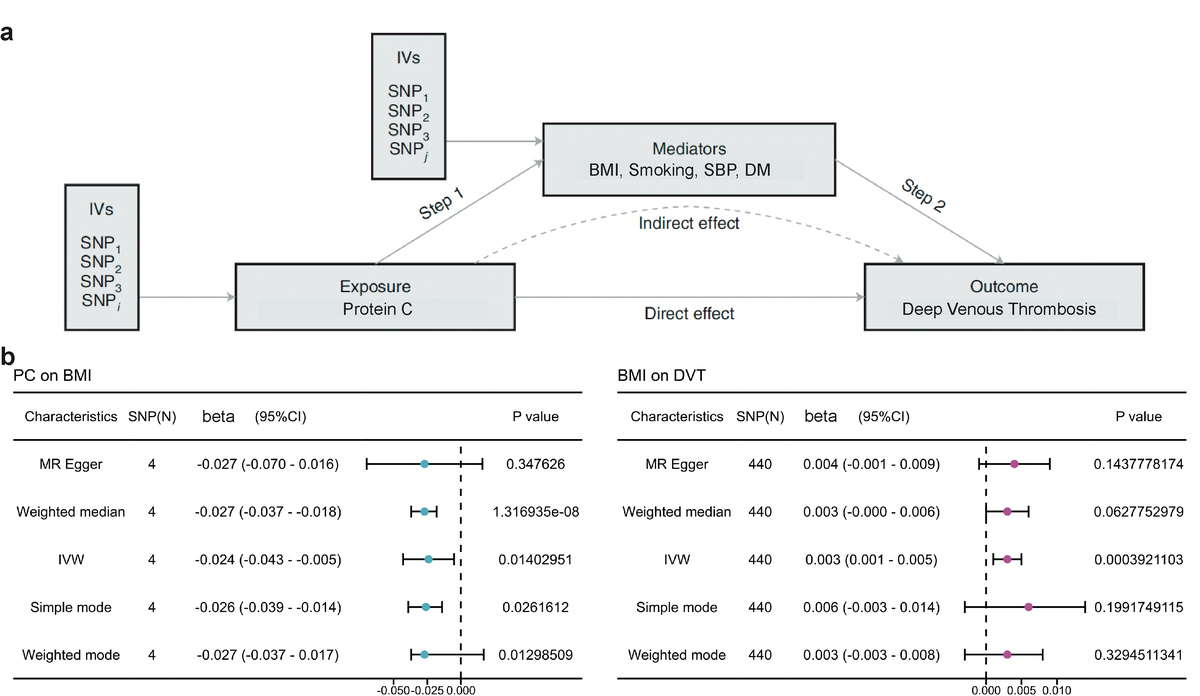
Mendelian randomization (MR) offers a valuable approach, and genetic variants may be associated with a lot of things; however, we assume that they affect the outcome on interest solely through utilizing genetic variants associated only with the risk factors of the exposure(s) of interest as instrumental variables [15]. In the context of coagulation profiles, identifying genetic variants solely linked to one component, such as protein C levels, is challenging due to the pleiotropic effects of these genes (which indicates that the genetic variation of protein C might affect other phenotypes apart from the outcome). Therefore, we adopted a mediation MR analysis to explore the potential causal association of anticoagulation factors, particularly relative protein C, with the risk factors of DVT, and further correlated these risk factors with DVT onset. Given the critical role of risk factors in DVT prevention and management [16], a two-step MR analysis was additionally conducted to investigate the mediating pathway from relative protein C, protein S and antithrombin-III against DVT through risk factor-related phenotypes.
Methods and materials
Study design and data sources
Study Design and Data Sources: Based on insights from prior research, we devised a two-sample (one to evaluate the gene-exposure relationship and the other to evaluate the gene-outcome relationship) Mendelian randomization (MR) approach to assess the causal impact of protein C, protein S and AT-III on DVT. Concurrently, we incorporated clinical risk factors associated with DVT incidence as mediating factors in the MR analysis, including body mass index (BMI), smoking, systolic blood pressure (SBP), diabetes mellitus (DM), and obesity. Single nucleotide polymorphisms (SNPs) serving as IVs were derived from previously published genome-wide association studies (GWAS) [17–19] and filtered for independence (linkage disequilibrium (LD) r2 < 0.001; clumping distance of 1000 kb). An F-statistic exceeding ten was considered robust for predicting the exposure of interest [20, 21]. To mitigate selection bias, datasets focused on individuals of European ancestry.
Data for exposure
SNPs linked to protein C and protein S at genome-wide significance (p < 5 × 10–8) were sourced from a GWAS encompassing 10,708 participants of European ancestry [22]. As this dataset lacked SNPs for antithrombin-III (AT-III), another dataset involving 3,301 participants of European ancestry was employed [23].
Data for outcome
GWAS summary data for DVT from two studies, comprising 484,598 participants of European ancestry and encompassing 9,587,836 SNPs, were utilized. Additionally, we utilized GWAS summary data on self-reported DVT in 337,159 participants of European ancestry from the Neale Lab consortium. We used the public GWAS dataset and ethical approval was exempted by the Institutional Review Board.
Statistical analysis
The two-sample MR method assessed causal effects, presenting results as odds ratios (ORs) with 95% confidence intervals (CIs). The procedure of harmonization of SNP exposure and outcomes was described previously [24]. Five MR approaches (random-effect inverse-variance weighted [IVW], weighted median, MR-Egger, simple mode, and weighted mode) addressed potential heterogeneity and horizontal pleiotropy [25], as genetic variants may be associated with several factors; however, it is assumed that they may affect the outcome of interest only through these genetic variants as instrumental variables. Sensitivity analyses compared causal estimates from various MR methods, including MR-Egger, penalized weighted median, simple mode, IVW, and weighted mode [26]. Forest plots and leave-one-out sensitivity analyses were employed as well [27]. Heterogeneity statistics were performed using scatter plots and Cochran’s Q tests between causal estimates from multiple genetic variants [28].
Validation with MR-IVW and expression quantitative trait loci (eQTL)s
To ensure the robustness of causal estimates derived from Mendelian randomization (MR) studies, adherence to three critical assumptions is imperative: (1) the genetic variants exhibit a robust association with the exposure, (2) the genetic variants remain free from any associations with potential confounders influencing the exposure–outcome link, and (3) the variants do not autonomously impact the outcome aside from their influence on the exposure. To satisfy the requirement of instruments being associated solely with the outcome through exposure, we meticulously excluded single nucleotide polymorphisms (SNPs) strongly correlated with the outcome. Subsequent to this, we harmonized the effects of SNPs on both exposure and outcome, ensuring that β values were aligned with the same alleles. Post-harmonization, palindromic SNPs with intermediate allele frequencies (> 0.42) and potential pleiotropic outliers underwent removal via a heterogeneity test, specifically modified Q statistics.
A comprehensive two-sample bidirectional MR analysis was then conducted to explore the relationships between each of genetic exposure of relative protein C, protein S, AT-III and DVT risk. The reversal MR is used to assess the effect of DVT outcome on the genetic variable of anticoagulation factors, which is a prerequisite for the mediating MR analysis. The multivariable MR-IVW method served to validate the causal associations of protein C with DVT, independent of other anti-coagulation factors. Subsequently, multivariable MR, utilizing the inverse-variance weighted approach, estimated the direct causal effects of protein C, protein S, and AT-III on DVT. The MR Steiger test was additionally employed to gauge the potential reverse causal impact of dietary compositions on DVT. Finally, validation of our findings was undertaken through the integration of expression quantitative trait loci (eQTLs) derived from genome-wide association study (GWAS) summary datasets, with each Ensemble Gene ID considered. A significance threshold of two-sided p < 0.05 was established for identifying causal associations for each analysis. For the multiple variable MR analysis, we applied the p threshold as 0.05/3 (Bonferroni correction) as three factors were included simultaneously. All statistical analyses were executed using R software version 3.6.2, with the TwoSampleMR package version 0.5.2 [29].
Exploration of potential mediators
Incorporating data from various GWAS sources, we sought potential mediators, focusing on obesity-related phenotypes with maximized sample sizes while meticulously avoiding any sample overlap (Supplementary Table SI). The selected potential mediators encompassed diverse information: body mass index (BMI) sourced from 461,460 individuals in the Genetic Investigation of Anthropometric Traits (ukb-b-19953), smoking data from 607,291 individuals in the GSCAN (ieu-b-4877), obesity-related information from 463,010 individuals in MRC-ICU (ukb-b-15541), systolic blood pressure (SBP) data from up to 757,601 individuals in the International Consortium of Blood Pressure Traits, and summary statistics for diabetes mellitus (DM) from up to 655,666 individuals (ebi-a-GCST006867).
Results
Univariable MR
Utilizing genetic variants associated with protein C, univariable MR analysis revealed a significant estimated relationship between relative protein C levels and DVT (Table I). The analysis demonstrated a 0.48% higher per one-standard deviation increment in relative protein C (IVW odds ratio (OR), 1.005; 95% confidence interval (CI): 1.002 to 1.008; p = 9.20e-04) (Table I). One standard deviation (SD) of relative PC has been reported to be approximately 0.530% of the protein C in UK Biobank (ukb-a-65; Supplementary Table SII). The scatter and forest plot of the protein C level and DVT relative risk correlation is shown in Figure 1. The detailed statistical information for instruments is listed in Table I. The Cochran’s Q statistic showed no pleiotropy between protein C and DVT (Q = 2.457, p = 0.293). Further, no outlier heterogeneity was detected by MR-PRESSO. MR-Egger also identified no directional pleiotropy (p = 0.084). The estimates were similar in size in weighted median (OR = 1.005; 95% CI: 1.04 to 1.007; p = 1.038e−13), weighted mode (OR = 1.005; 95% CI: 1.04 to 1.007; p = 4.775e−3) and simple mode (OR = 1.004; 95% CI: 1.002 to 1.006; p = 1.97e−2), supporting a relative risk effect of relative PC against DVT. The CIs of MR-Egger were wider (OR = 1.008; 95% CI: 1.006 to 1.011; p = 0.02) than those of other methods. Additionally, the protein C level was almost not associated with arterial embolism genetically (finn-b-I9_ARTEMBTHR, data not shown).
Table I
MR results for the relationship between anti-coagulation compositions and DVT
| Method | Number of SNPs | F | OR (95% CI) | P-value |
|---|---|---|---|---|
| Vitamin K-dependent protein C: | ||||
| MR Egger | 4 | 11 | 1.008318 1.005742 1.010901 | 2.394115e-02 |
| Weighted median | 4 | 1.005254 1.003867 1.006644 | 1.038157e-13 | |
| Inverse variance weighted | 4 | 1.004755 1.001940 1.007577 | 9.198350e-04 | |
| Simple mode | 4 | 1.004359 1.002484 1.006237 | 1.974418e-02 | |
| Weighted mode | 4 | 1.005336 1.003953 1.006722 | 4.774844e-03 | |
| Vitamin K-dependent protein S: | ||||
| MR Egger | 4 | 21 | 0.9994879 0.9909100 1.0081400 | 0.91791552 |
| Weighted median | 4 | 0.9975924 0.9948052 1.0003874 | 0.09127953 | |
| Inverse variance weighted | 4 | 0.9965908 0.9920104 1.0011924 | 0.14622656 | |
| Simple mode | 4 | 0.9918502 0.9861492 0.9975841 | 0.06884925 | |
| Weighted mode | 4 | 0.9998972 0.9971954 1.0026063 | 0.94530664 | |
| Antithrombin-III: | ||||
| Wald ratio* | 1 | 10 | 1.001473 0.9976032 1.005358 | 0.4561531 |
Figure 1
Mendelian randomization plots for relative protein C and DVT relationship: A – in this scatter plot, the effects of single nucleotide polymorphisms (SNPs) on relative protein C level are juxtaposed against their effects on deep venous thrombosis (DVT). Each line’s slope corresponds to the estimated Mendelian randomization (MR) effect per method, with data presented as raw β values and accompanied by 95% confidence intervals (CIs). B – The forest plot shows individual and combined SNP MR-estimated effect sizes. These estimates represent the log odds for DVT per one standard deviation increase in mean relative protein C level, and error bars depict 95% CIs
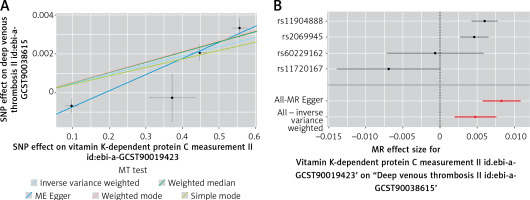
Further, leave-one-out analysis showed that almost no single SNP is involved in this relationship, suggesting an overall positive relationship between protein C and DVT (Supplementary Figure S1). We also found no reverse causality by the MR Steiger test (Supplementary Figure S2). The univariable MR estimates were not statistically significant for the effect of relative protein S (IVW OR = 0.997; 95% CI: 0.992 to 1.001; p = 0.146) or relative antithrombin-III (IVW OR = 1.001; 95% CI: 0.998 to 1.005; p = 0.456) on DVT (Table I).
Multivariable MR
Considering the interrelated nature of risk factors, a multivariable MR analysis was conducted. The effect estimated for relative protein C on DVT remained consistent with univariable estimates. CIs for multivariable MR-Egger were comparable, and the analysis provided no conclusive evidence for horizontal pleiotropy. The effects of relative protein S and relative AT-III on DVT were not significant (Table II). The effect estimated for relative protein C level on DVT was comparable to the univariable IVW estimate (univariable IVW OR = 1.005; 95% CI: 1.002 to 1.008; p = 9.20e-04; multivariable IVW OR = 1.005; 95% CI: 1.001 to 1.009; p = 0.005, which passed the Bonferroni correction). The CIs of multivariable MR-Egger were similar to those of multivariable IVW (Table II). The multivariable MR-Egger intercept analysis did not provide conclusive evidence for horizontal pleiotropy (p = 0.152). The multivariable MR estimates for relative PS level (IVW OR = 0.996; 95% CI: 0.991 to 1.001; p = 0.088) and relative AT-III (IVW OR = 0.999; 95% CI: 0.990 to 1.009; p = 0.882) were not significant (Supplementary Figure S3).
Table II
Multivariable MR analysis estimating the effect of relative PC on DVT, conditioning on other anti-coagulation compositions
Effect of DVT on relative protein C level
In the reverse direction, bidirectional MR analyses exploring the causal effect of DVT as the genetic exposure on anti-coagulation factors as the genetic outcome found no evidence of causal relationships with relative protein C (IVW β = 153.536; 95% CI: 1.292e-05 to 1.825e+09; p = 0.545), relative protein S (IVW β = 3.552; 95% CI: 0.445 to 28.319; p = 0.231) or relative AT-III (IVW β = 2.427; 95% CI: 4.022e-03 to 1.464e+03; p = 0.786) (Table III). Further, no outlier heterogeneity was detected by MR-PRESSO. The weighted median, the weighted mode, and MR-Egger showed similar results (data not shown). Leave-one SNP-out analysis also identified no leverage points (data not shown). The relationship between protein C, protein S, antithrombin-III and DVT was lastly validated with eQTLs. Again, we found that only protein C had a positive causal association with DVT.
Table III
Bidirectional MR results for the relationship between DVT and anti-coagulation compositions
Mediation analysis
Given the crucial role of risk factors in venous thrombosis prevention [16], a two-step MR analysis was performed to investigate obesity-related measurements as potential mediators of the protective effect of relative protein C on DVT. The analysis identified a causal relationship between relative protein C and BMI, with increased protein C associated with decreased BMI. Subsequent analysis confirmed a causal effect of BMI on DVT, with a mediated proportion of 11.4% (Figure 2 A). Firstly, exposure instruments such as protein C were employed to investigate the causal effect on the potential risk factors (mediators). Detailed information about risk factors for the SNPs with DVT is listed in Supplementary Table SIII. Among five potential mediators, we found a causally negative relationship only between protein C level and BMI (IVW β = –0.024; 95% CI: –0.043 to –0.005; p = 0.014) (Figure 2 B, Supplementary Table SIV). Secondly, we investigated the causal role of the mediators in DVT risk using genetic exposure for the risk factors. We identified a causal role of BMI (IVW OR = 1.003; 95% CI: 1.001 to 1.005; p = 0.0004) in DVT (Figure 2 B and Supplementary Table SV). The results from other MR methods were consistent. We then employed a multivariable MR (MVMR) to assess the role of BMI on DVT against other obesity-related phenotypes. The MVMR effect estimated for BMI on DVT was similar to protein C (OR = 1.004; 95% CI: 1.002 to 1.006; p = 0.0002) (Supplementary Table SVI), which also passed the Bonferroni Correction. The multivariable MR estimates of the effects of other measures on DVT were not significant (data not shown). Finally, we assessed the indirect effect of protein C level on DVT via BMI and found that the mediation effect of BMI was 0.003 (95% CI: 0.001 to 0.004; p = 5.552e-05) with a mediated proportion of 11.4% (95% CI: 2.3% to 79.2%) (Table IV).
Table IV
Mediation effect of protein C on DVT via BMI
Figure 2
Mediation analysis of relative protein C effect on DVT via potential mediators: A – a two-step MR analysis framework is illustrated. Step 1 involves estimating the causal effect of the exposure (relative protein C) on potential mediators, while Step 2 assesses the causal effect of these mediators on DVT risk. The ʽDirect effectʼ indicates the impact of relative protein C level on DVT risk after adjusting for the mediator, while the ʽIndirect effectʼ signifies the influence of relative protein C level on DVT risk through the mediator. Instrumental variables (IVs) are employed in this analysis. B – Summary MR estimates derived from the inverse-variance weighted (IVW), weighted median, weighted mode, and MR-Egger methods are presented for the effect of relative protein C level on BMI (left) and the effect of BMI on DVT (right). The error bars denote 95% CIs, and all statistical tests were two-sided, considering p < 0.05 as significant.
Validation analysis
To validate the results, we used the protein QTLs (pQTLs) for protein C, protein S and AT-III to associate them with the DVT outcome and found that only protein C was positively correlated with DVT (Supplementary Figure S4).
Discussions
This pioneering study leverages the Mendelian randomization (MR) approach to discern the causal links between protein C, protein S, antithrombin-III, and the risk of DVT. Our extensive multivariable MR (MVMR) investigation established a positive causal association between genetically predicted protein C levels and DVT risk. In contrast, coagulation traits such as protein S and antithrombin-III exhibit no discernible causal association with DVT. Additionally, a mediation analysis revealed that the influence of relative protein C on DVT is partially mediated by BMI, although the indirect effect is notably smaller than the total effect.
Factor V Leiden, marked by inadequate anticoagulant response to activated protein C, poses an elevated risk for venous thromboembolism [30]. Our findings underscore the complexity of clinical decisions for asymptomatic Factor V Leiden carriers, suggesting that long-term anticoagulation may not be universally recommended. Notably, conditions featuring low protein C or impaired function, such as protein C deficiency and antiphospholipid syndrome, increase the risk of DVT. Protein S, a natural anticoagulant akin to protein C, mirrors the relationship with DVT, with both proteins crucial in regulating blood clotting [30]. When there is a deficiency or dysfunction of protein C, the body’s ability to control blood clotting is impaired. This can lead to a higher propensity for the formation of DVT, especially in situations where the risk of clot formation is already elevated (e.g., surgery, immobility, pregnancy). Conditions associated with low levels of protein C or impaired protein C function are considered hypercoagulable states. These conditions can include protein C deficiency, protein S deficiency, and antiphospholipid syndrome, among others. In these situations, the risk of DVT and other thrombotic events is increased. Protein S is another natural anticoagulant protein in the body that, like protein C, plays a crucial role in regulating blood clotting. The relationship between protein S and DVT is similar to that of protein C.
Our robust sensitivity analyses, employing various MR methods, affirm an unconfounded effect of plasma protein C levels on DVT risk. This contradicts prior studies hinting at a link between protein C deficiency and adverse long-term post-acute DVT outcomes, which proposed that reduced initial protein C was correlated with worse long-term survival after acute DVT [1, 2,6]. However, these studies were complicated by limitations, such as small sample sizes and statistical errors, emphasizing the importance of our large-scale, genetically informed approach. Notably, our bidirectional analysis dismisses the notion that a genetic predisposition to DVT causally influences protein C levels, challenging previous assumptions.
Various biological mechanisms are proposed to elucidate the potential detrimental role of protein C (PC) in the development of venous thrombosis. In this study, a comprehensive two-step Mendelian randomization (MR) for mediation analysis revealed that the adverse impact of elevated relative protein C on deep venous thrombosis (DVT) risk is partially mediated by body mass index (BMI), albeit with an indirect effect that is less pronounced than the total effect. In the initial MR step, univariable MR identified a causal link between relative protein C levels and BMI, indicating that increased relative protein C is associated with decreased BMI. This finding aligns with prior research demonstrating a linear increase in activated protein C (APC) resistance with rising BMI, particularly observed in men. Coagulation factor VIII (FVIII) levels were implicated in mediating the relationship between BMI and decreased APC ratio [12]. The relationship between protein C and BMI is complex and not fully understood.
The subsequent MR step furnished evidence supporting a genetically determined higher BMI as a contributing factor to increased odds of DVT. Consistent with previous MR studies, which highlighted the causal relationship between BMI and DVT or related thrombosis phenotypes [16, 31], our second-step mediation analysis aligns with these findings. Notably, Christiansen et al. observed elevated DVT risk in individuals with BMI in the upper tertile compared to the lowest tertile, reinforcing the causal role of BMI in DVT pathogenesis [12]. Contrary to expectations, no significant causal associations were observed for smoking, obesity, SBP, and DM with DVT risk, underscoring the prominence of the causal effects of protein C and BMI over other factors.
Protein C is an important protein in the body that functions as an anticoagulant, which means it helps to prevent blood clots from forming. It is a vitamin K-dependent serine protease enzyme, which is activated to its active form, activated protein C (APC), by the thrombin-thrombomodulin complex on endothelial cell surfaces. APC, in turn, degrades Factor Va and Factor VIIIa, which are required for blood clotting.
For its prediction role, protein C deficiency can lead to an increased risk of venous thromboembolism, including DVT and pulmonary embolism (PE). Patients with low levels of protein C may be predisposed to these conditions. Monitoring the levels of protein C can be useful in diagnosing and predicting the progression of disseminated intravascular coagulation. Monitoring protein C levels can also help in managing anticoagulation therapy to prevent both bleeding and thrombosis.
Activation of protein C in endothelium, which co-operates with thrombin pathways, can protect against thrombotic events. However, in inflammation, infection, malignancy, obesity, and even pregnancy, excess protein C might overcome its anticoagulant effect in the activated form and augment thrombosis. Therefore, it is important to note that protein C levels should be interpreted in the context of the patient’s overall clinical picture and other laboratory results. The predictive value of protein C levels can vary depending on the specific disease and individual patient characteristics.
It is important to note that the relationship between protein C and BMI is still an active area of research, and the specific mechanisms and clinical implications are not fully understood. Additionally, individual responses to obesity can vary widely. People with obesity should be aware of the potential risks associated with clotting disorders and consult with healthcare providers for appropriate monitoring and management, especially if they have other risk factors or a personal or family history of clotting problems.
Admittedly, our study has limitations that warrant careful interpretation, which need to be addressed. Potential horizontal pleiotropy, introducing bias into causal effect estimates, was addressed through rigorous methods, including outlier removal using MR-PRESSO and MR-Egger approaches. Notably, neither heterogeneity nor pleiotropy influenced the causal effect in our analyses. Canalization effects, modifying the association of genetically predicted protein C, protein S, and antithrombin-III profiles with DVT during development, were considered. Our MR analyses focused on evaluating not only heritable variations but also the causal effects of environmental exposures. Pregnancy as a DVT risk factor, gender-specific associations, and environmental factors were acknowledged as crucial components of DVT pathogenesis, albeit with inherent complexities. Although the terminology “causal relationship” was used, some findings may be just associations due to the limitation of the MR itself, and actual causal effect needs to be explored with in-vitro and in-vivo experiments in future studies. In addition, the finding regarding the positive relationship between genetic exposure of protein C and DVT onset should be interpreted carefully in a clinical session. As the effect size of this relative relationship is small, for the univariable MR analysis, it was 1.002 to 1.008 (p = 0.20e-04) while for the MVMR analysis, the CI was 1.001 to 1.009 (p = 0.005). Although both p values were smaller than the Bonferroni correction threshold, the effect size was quite small and needed to be carefully interpreted. In addition, the effect size for the mediating effect is quite wide, from 2% to 79%, and this might be due to the statistical limitation itself, which needs to be validated in a bigger population study.
In conclusion, our MVMR and mediating analyses offer genetic evidence supporting a positively potential causal association between protein C levels and increased DVT risk, with BMI playing a modest mediating role. The potential implications of our results for DVT prevention policies underscore the need for validation in well-powered randomized clinical trials.