Introduction
Psoriasis is an inflammatory disease with visible manifestations that has no cure [1]. In 2016, psoriasis was estimated to affect 65 million people around the world [2]. Epidemiological studies have found that psoriasis is associated with metabolic syndrome (MS). Metabolic syndrome is typically defined based on at least three of the following medical conditions: obesity, dyslipidemia, hypertension, and hyperglycemia [3]. In Poland among 810 postmenopausal women, 34% were diagnosed with both the disorders [4]. In India, MS was significantly more common in psoriatic patients than in controls 42 (28%) vs. 9 (6%), and psoriatic patients also had a significantly higher prevalence of MS [5]. A study based on a large population in the UK by Langan et al. demonstrated that psoriasis and MS have a dose-response association. The incidence of MS is 22% in patients with mild psoriasis, 56% in moderate psoriasis, and 98% in severe psoriasis [6]. Additionally, a randomized controlled trial (RCT) suggested that treatment of MS, such as diet control, can improve the condition of psoriasis [7]. Another RCT study showed that psoriasis patients with MS can improve the condition of psoriasis with the treatment of insulin sensitizers [8].
It is believed that psoriasis pathogenesis is caused by a combination of innate and acquired immunity. When some pathogenic factors break through the skin, antibacterial peptides can destroy the body’s natural immune tolerance to its DNA/RNA and bind to form an immune complex. The complexes stimulate the dendritic cells, which in turn produce the cytokine interferon-α (IFN-α) mediated by TLR8, leading to the onset and persistence of psoriasis [9]. Psoriasis and MS are both chronic inflammatory diseases and the inflammatory response may be one common pathogenesis of them. Previous research has shown that serum C-reactive protein (CRP) levels are significantly higher in psoriasis patients with MS than psoriasis patients without MS [10]. The inflammatory component of psoriasis may affect MS components, and the mixture of inflammatory molecules and hormones of MS might impact psoriasis as well, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-17 and IL-18 [11–13]. Boehncke et al. indicated that when psoriasis develops to a more severe degree, it forms systemic inflammation and causes insulin resistance, which in turn triggers endothelial cell dysfunction, leading to atherosclerosis and finally myocardial infarction or stroke [14].
Although previous studies have shown a significant association between psoriasis and MS, new studies have been published, and negative results were reported [15–17]. Our study included studies with non-psoriasis groups, which better assessed the association between psoriasis and MS. The objective of this study was to evaluate the relationship between psoriasis and MS.
Material and methods
Search strategy and selection criteria
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched electronic databases (PubMed, EBSCO, Elsevier, Springer, Wiley, and Cochrane) for studies published up to November 2, 2018 from the database inception and used the Mesh terms “psoriasis/metabolism” or “psoriasis/complications”. The inclusion criteria were as follows: (1) case-control or cross-sectional study, (2) association between MS and psoriasis reported by odds ratio (OR) and 95% confidence interval (CI), and (3) adult patients. The exclusion criteria were as follows: (1) duplication of research literature, (2) systematic reviews and meta-analyses, (3) studies not reported in English, (4) studies with no control group, and (5) OR of MS and psoriasis were not reported. We contacted the corresponding author in case we failed to obtain an article.
Data extraction
Ju Qiao and Qiannan Jia were in charge of data extraction. The data included the first author’s name, year of publication, type of study, number of patients (cases) and controls, age (mean), gender (male/female), diagnostic criteria for MS and psoriasis, adjusted OR reported or not, and ethical considerations.
Statistical analysis
We evaluated the association between MS and psoriasis using ORs and 95% CIs. The statistical heterogeneity of the study was estimated using the Q test and I2 index. If there was no statistically significant heterogeneity (p > 0.1 and I2 < 50%), a pooled effect was calculated with a fixed-effects model, whereas a random-effects model was employed otherwise (p < 0.1 or I2 > 50%). We used Begg’s test and plot to assess the possible publication bias in the included studies. When heterogeneity was found, meta-regression was performed to assess whether publication year, study type (case-control or cross-sectional study), diagnostic criteria for MS (NCEP ATP III criteria or not), diagnosis of psoriasis (clinical diagnosis or not), adjusted OR reported or not, and source of controls were potential confounding factors. Subgroup analyses were conducted according to the result of the meta-regression. All statistical analyses were conducted using Stata version 11.0 (Stata Corp LLC, College Station, TX, USA). P-values less than 0.05 were considered statistically significant.
Results
Characteristics of included studies
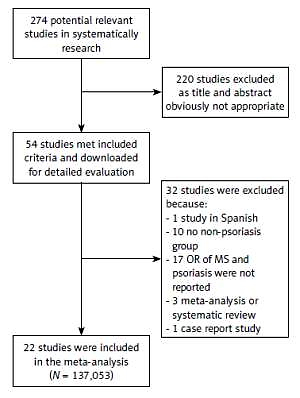
We obtained 274 studies by systematic screening of the literature; 220 studies were excluded because they were found to be irrelevant after title and abstract reading. After further screening, 32 studies were excluded (one study was in Spanish, 10 studies had no control group, 17 studies did not report ORs, 3 studies were meta-analyses or systematic reviews and 1 was a case report study). Finally, twenty-two studies [15–35] with a total of 137,053 participants were included in the meta-analysis (Figure 1, Table I).
Table I
Characteristics of studies included in the meta-analysis
| Author | Year | N | Study type | Diagnosis of MS | Diagnosis of psoriasis | Age | M/F | Case | Control | Source of control | Adjusted OR | Ethics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gui et al. [39] | 2018 | 2577 | Cross-sectional | Criteria recommended by the Chinese Diabetes Society | Clinical diagnosis | 41.29 | 1641/936 | 859 | 1718 | Hospital non-PSA subjects | Age and gender | Ethics committee approved |
| Milcić et al. [15] | 2017 | 407 | Cross-sectional | NCEP-ATP III | Clinical diagnosis | 49.60 | 213/194 | 244 | 163 | Hospital non-psoriasis subjects | Age and gender | Written informed consent |
| Koku Aksu et al. [16] | 2017 | 477 | Cross-sectional | NCEP-ATP III | Clinical diagnosis | 44.69 | 237/240 | 300 | 177 | Hospital non-psoriasis subjects | No | Ethics committee approved |
| Sharma et al. [18] | 2016 | 200 | Case-control | NCEP-ATP III | Clinical diagnosis | 44.11 | 130/70 | 100 | 100 | Population non-psoriasis subjects | No | Written informed consent |
| Kothiwala et al. [19] | 2016 | 280 | Cross-sectional | NCEP-ATP III | Self report | 37.00 | 199/81 | 140 | 140 | Hospital non-psoriasis subjects | No | Written informed consent & IRB approved |
| Meziane et al. [20] | 2016 | 450 | Case-control | International Diabetes Foundation | Clinical diagnosis | 40.80 | – | 150 | 300 | Hospital non-psoriasis subjects | No | Ethics committee approved |
| Itani et al. [21] | 2016 | 300 | Case-control | NCEP-ATP III | Clinical diagnosis | 42.03 | 135/165 | 150 | 150 | Population non-psoriasis subjects | Smoking | Written informed consent & IRB approved |
| Raznatovic-Durovic et al. [22] | 2016 | 227 | Case-control | NCEP-ATP III | Clinical diagnosis | 46.50 | 100/127 | 101 | 126 | Hospital non-psoriasis subjects | Age, gender, smoking, physical activity | Written informed consent |
| Miller et al. [23] | 2015 | 14876 | Cross-sectional | NCEP-ATP III | Questionnaire | – | – | 860 | 14016 | Population non-psoriasis subjects | Age, gender and smoking | Written informed consent |
| Parodi et al. [24] | 2014 | 734 | Cross-sectional | NCEP-ATP III | Clinical diagnosis | 53.79 | 403/331 | 390 | 344 | Hospital non-psoriasis subjects | Age, gender et al. | Written informed consent |
| Damevska et al. [25] | 2013 | 244 | Case-control | NCEP-ATP III | Clinical diagnosis | 51.75 | 104/140 | 122 | 122 | Population non-psoriasis subjects | No | Written informed consent |
| Vaya et al. [17] | 2012 | 192 | Case-control | NCEP-ATP III | Clinical diagnosis | 51.27 | 98/94 | 91 | 101 | Population non-psoriasis subjects | Age gender et al. | Ethical guidelines approved |
| Arias-Santiago et al. [26] | 2012 | 133 | Case–control | NCEP-ATP III | Clinical diagnosis | – | 70/63 | 72 | 61 | Population non-psoriasis subjects | No | Written informed consent |
| Langan et al. [27] | 2012 | 44715 | Cross-sectional | NCEP-ATP III | Questionnaire | 45-65 | 21385/23330 | 4065 | 40650 | Population non-psoriasis subjects | Age, gender | IRB approved |
| Love et al. [28] | 2011 | 2456 | Cross-sectional | NCEP-ATP III | Self-report | 39.00 | 1183/1273 | 71 | 2385 | Population non-psoriasis subjects | Age, gender, race/ethnicity, smoking status and C-reactive protein levels | Written informed consent & IRB approved |
| Mebazaa et al. [29] | 2011 | 380 | Case-control | NCEP-ATP III | Clinical diagnosis | 47.62 | 180/200 | 164 | 216 | Population non-psoriasis subjects | No | Written informed consent |
| Takahashi et al. [30] | 2010 | 305 | Case-control | Japan Committee for the Diagnostic Criteria of Metabolic Syndrome | Clinical diagnosis | 55.17 | 218/87 | 151 | 154 | Population non-psoriasis subjects | No | No mention |
| Chen et al. [31] | 2009 | 77 | Case-control | NCEP-ATP III | Clinical diagnosis | 57.48 | 54/23 | 40 | 37 | Population non-psoriasis subjects | No | IRB approved |
| Cohen et al. [32] | 2008 | 65532 | Case-control | Customize standard | Clinical diagnosis | 48.87 | 31812/33720 | 16,851 | 48,681 | Population non-psoriasis subjects | Age and gender | No mention |
| Chen et al. [33] | 2008 | 158 | Case-control | NCEP-ATP III | Clinical diagnosis | 56.49 | 112/46 | 77 | 81 | Population non-psoriasis subjects | No | IRB approved |
| Gisondi et al. [34] | 2007 | 672 | Case–control | NCEP-ATP III | Clinical diagnosis | 62.95 | 310/362 | 338 | 334 | Population non-psoriasis subjects | Age and gender | Written informed consent |
| Sommer et al. [35] | 2006 | 1625 | Case-control | WHO criteria | Clinical diagnosis | 57.03 | 786/839 | 581 | 1,044 | Hospital non-psoriasis subjects | Age and gender | No mention |
Of the 22 included studies, 14 were case-control studies and the others were cross-sectional studies. In the diagnosis of MS, different criteria were used: the NCEPATP III was used in 18 studies, one study used the International Diabetes Foundation criteria [20], one used the Japanese Committee for the Diagnostic Criteria of Metabolic Syndrome, one used criteria recommended by the Chinese Diabetes Society, one used the WHO criteria and one was a customized standard [35]. In seventeen studies psoriasis was diagnosed clinically, two were based on self-report, and three used questionnaires. In the included studies, informed consent was obtained as well as approval by an institutional review board, but three studies did not describe ethical considerations.
Quantitative synthesis
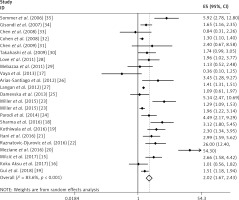
In this meta-analysis, we found that psoriasis was associated with MS and the combined OR (95% CI) was 2.02 (1.67–2.43) with p < 0.001. The results are shown in Figure 2.
This analysis was subject to significant heterogeneity (I2 = 83.60%, p < 0.001). The data from the meta-regression are shown in Table II, and only the source of controls may have influenced the heterogeneity in our study.
Table II
Results of meta-regression
| Variables | b | Se | t | P-value | 95% CI | |
|---|---|---|---|---|---|---|
| LCI | UCI | |||||
| Type of study | –0.77 | 0.41 | –1.85 | 0.08 | –1.64 | 0.11 |
| Diagnosis of MS | 0.53 | 0.36 | 1.48 | 0.16 | –0.22 | 1.28 |
| Diagnosis of psoriasis | 0.97 | 0.49 | 1.96 | 0.07 | –0.08 | 2.01 |
| Adjusted OR | 0.16 | 0.30 | 0.54 | 0.60 | –0.48 | 0.80 |
| Source of control* | –1.02 | 0.35 | –2.94 | 0.01 | –1.75 | –0.29 |
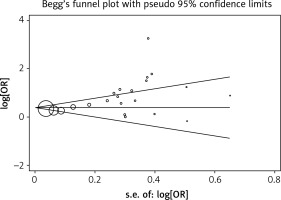
Begg’s test was applied to assess the included literature’s publication bias in the this study and no significant publication bias was found; the Egger’s test p-value was 0.119. The result is shown in Figure 3.
Discussion
We evaluated the relationship between psoriasis and MS using combined OR by meta-analysis. In our study, 22 case-control or cross-sectional studies were included, with 25,953 psoriasis patients and 111,100 controls. The age range of the participants was 37.00–65.00 years. Psoriasis was found to be associated with MS from the meta-analysis (OR = 2.02; 95% CI: 1.67–2.43) and is similar to the published meta-analysis study [36].
To the best of our knowledge, this is the first study to discuss the factor of heterogeneity in a meta-analysis of psoriasis and MS. We assessed potential factors including study type, MS diagnostic criteria, diagnosis of psoriasis, reported adjusted OR, and the source of non-psoriasis controls in the meta-regression. Of the five factors, only the source of controls significantly affected the heterogeneity (p = 0.01). In studies with matching non-psoriasis controls, the heterogeneity no longer existed according to the further subgroup analysis. The control group was established to better analyze the relationship between psoriasis and MS. In observational studies, the selection of control groups can significantly affect the results. Studies with matched control groups can be more accurate in describing the relationship between psoriasis and MS. Therefore, in our analysis, psoriasis was significantly associated with MS, especially in the studies with matched non-psoriasis control groups.
The underlying mechanism of the relationship between psoriasis and MS is complex. Psoriasis is an inflammatory disease involving many factors. One of them is high-sensitivity C-reactive protein (hs-CRP). Hs-CRP is a sensitive, nonspecific systemic inflammatory marker. Danielsen et al. found that psoriasis patients with elevated hs-CRP levels showed negative metabolic status, especially in women [37]. One study indicated that the mechanism of the association of psoriasis with atherosclerosis (one disease that may be caused by MS) was similar to the association of atherosclerosis with systemic lupus erythematosus [38]. Previous hospital-based cross-sectional studies have indicated that the duration of psoriasis may be a risk factor for MS [39, 40]. The chronic inflammatory level of psoriasis might give the explanation. Jensen et al. found that low-energy diet treatment led to a clinical improvement in the psoriasis area and severity index [41]. Therefore, the treatment of MS in patients with co-existing psoriasis could potentially lower the onset and severity of psoriasis.
IL-17 is a pro-inflammatory cytokine which is mainly secreted by activated Th-17 cells and the differentiation of Th-17 depends on IL-23 [42]. There is increasing evidence showing that theIL-23/Th17 signaling pathway is involved in the pathogenesis of psoriasis [43] and MS [11]. Higher levels of IL-17, IL-23 and TNF-α were observed in patients with MS accompanying psoriasis compared to patients with psoriasis without the features of this syndrome [44]. Rasmy et al. found that the IL-18 expression in the skin lesions was significantly lower than lesional skin after therapeutic interventions [45]. Recently, novel IL-17 and IL-23 antagonists for the treatment of psoriasis were approved by the Food and Drug Administration [46]. Many approaches in MS management may also treat psoriasis, such as omega-3 fatty acids and vitamins [47]. Clinical studies have confirmed that vitamin D can effectively reduce the levels of IL-17 and IL-17-expressing cells in psoriasis lesions [48]. In MS patients, taking vitamin D decreased systolic blood pressure by 3.7% [49].
Psoriasis, especially in combination with MS, has many complications. Akcali et al. reported that MS was more frequently identified in Turkish patients with psoriasis than in controls. Psoriasis patients with MS have a high risk of cardiovascular disease and believe that improvement of MS was beneficial to reducing the risk of cardiovascular disease [50].
This meta-analysis has several limitations. This study included 22 observational studies with non-psoriasis control groups, which provides better evidence to assess the relationship between psoriasis and MS. We also chose five factors to assess the origin of heterogeneity, and found that the source of controls in each study was a major determinant of heterogeneity. However, this meta-analysis may indicate, though not definitely, the association between psoriasis and MS. A relative cohort or experimental study needs to be conducted to explore the relationship between psoriasis and MS, and the underlying mechanism.
In conclusion, psoriasis was associated with MS according to our meta-analysis. The source of the control group had a significant effect on the combined OR value.