Bone mineral density (BMD) refers to the amount of mineral content per unit area of bone, serving as a clinical indicator of skeletal development and an assessment of bone calcium content [1]. Low BMD increases bone fragility, which is closely associated with a higher risk of fractures, potentially leading to increased bed rest and significantly impacting the quality of life [2]. Estrogen significantly impacts female BMD. Fluctuations in estrogen levels can affect bone turnover rates, which in turn influence BMD [3].
Throughout a woman’s life, estrogen levels fluctuate due to various reproductive characteristics including onset of menstruation, initial sexual encounter, pregnancy, and climacteric. Consequently, female reproductive characteristics including age at menarche (AAM), age at first birth (AFB), age at first sexual intercourse (AFS), and age at natural menopause (ANM) may increase the risk of postmenopausal BMD loss [4]. A study conducted in the United States revealed that among middle-aged and elderly individuals aged 50–80 (a period during which women are typically postmenopausal, with the lowest estrogen levels), 36.9% of women and 7.2% of men were affected by low BMD [5]. The risk of developing low BMD is significantly higher in women compared to men [5]. Some studies suggest that postmenopausal low BMD may be associated with female reproductive characteristics [6], though this remains a contentious issue.
Exploring the exact relationship between female reproductive characteristics and postmenopausal low BMD could be key for future predictions of low BMD in women. Additionally, this understanding could provide valuable insights for the prevention of low BMD.
Mendelian randomization aims to overcome the limitations of traditional observational studies by reducing confounding bias, reverse causation, and measurement error, thereby providing more accurate and reliable causal inferences. It serves as an important tool for understanding causal relationships in epidemiological research, especially in scenarios where randomized controlled trials are not feasible [7].
This study utilized the MR method to investigate the association between female reproductive characteristics (AAM, AFB, AFS, ANM) and BMD. By identifying the factors influencing low BMD, this research aimed to provide predictive indicators for the occurrence of postmenopausal low BMD.
Material and methods. Study design. In our study, female reproductive characteristics served as the exposure factor, and IVs consisted of single nucleotide polymorphisms (SNPs) displaying robust correlations with female reproductive characteristics (AAM, AFB, AFS, and ANM). BMD served as the outcome variable in this study.
GWAS data sources. GWAS data related to SNPs were obtained from publicly available GWAS. Genetic summary data for AAM, AFB, AFS, and ANM were sourced from the UK Biobank (http://www.nealelab.is/uk-biobank), encompassing 279,470, 397,338, 542,901, and 143,819 individuals of European descent, respectively. For BMD, summary genetic data were retrieved from a meta-GWAS study, which included 365,403 individuals of European ancestry. Further information on the data is provided in Table I.
Table I
Overview of the data sources of phenotype used in the MR study
Selection of IVs. We selected SNPs significantly associated with female reproductive traits, including AAM, AFB, AFS, and ANM, with a screening threshold of genome-wide significance (p < 5 × 10−8). To ensure that all SNPs used for exposure analysis were not in linkage disequilibrium (LD), we performed an LD analysis, considering an LD correlation coefficient (r2 < 0.001) and a base pair distance greater than 10,000 kb between two SNPs. Next, we removed palindromic and ambiguous SNPs. We then assessed the selected SNPs using the LDlink website (https://ldlink.nih.gov/, accessed on May 10, 2024). Subsequently, we excluded potential confounding SNPs associated with OP (including type 2 diabetes, smoking, drinking, osteoarthritis, BMI, education) and those directly related to BMD. We selected SNPs with an F-statistic greater than 10 for further analysis.
MR analysis. To investigate the possible causal relationship between female reproductive factors (AAM, AFB, AFS, and ANM) and BMD, we performed a MR analysis using four methods: IVW, MR-Egger, weighted median, and weighted mode. These methods are considered the most robust and scientifically sound approaches commonly used to ensure the reliability of MR analyses.
Sensitivity analysis. Sensitivity analysis consists of two components: the heterogeneity test and the pleiotropy test. To assess potential heterogeneity, Cochran’s Q test was applied to examine any variations in our study [8]. MR-Egger regression was employed to assess pleiotropy [9]. Furthermore, we performed a “leave-one-out” analysis to determine whether any individual SNP had an impact on the results [10].
Statistical analysis. All analyses were performed utilizing the “two-sample MR” package in the R programming language (version 4.2.3). P < 0.05 was deemed statistically significant.
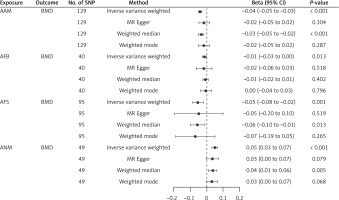
Results. Selection of IVs. After removing outlier SNPs detected by the MR radial method, we finally obtained 129 SNPs in AAM, 40 SNPs in AFS, 95 SNPs in AFB, and 49 SNPs in ANM. The F statistics for all the IVs exceeded 10, suggesting the absence of weak instrument bias in our MR analysis. Detailed information regarding these SNPs is provided in Supplementary Table SI.
MR analysis. The fixed-effects IVW method showed that the beta and corresponding 95% confidence intervals (CI) for various exposures are as follows: AAM: beta = –0.040, 95% CI = [–0.052, –0.029], p = 4.94 × 10−5 ; AFB: beta = –0.014, 95% CI = [–0.026, –0.003], p = 1.34 × 10−2 ; AFS: beta = –0.052, 95% CI = [–0.083, –0.022], p = 8.26 × 10−4 and ANM: beta = 0.049, 95% CI = [0.032, 0.065], p = 8.07 × 10−9 (Figure 1). As indicated by the finding, we identified a causality linking female reproductive characteristics (AAM, AFB, AFS, and ANM) with BMD. Other methods demonstrated that these relationships persisted (Figure 1).
Sensitivity analysis. This study showed no evidence of pleiotropy (Table II), indicating that IVs do not affect outcome via the confounding factors. No heterogeneity was observed in our study for AAM (p = 0.160, Q = 142.775), AFB (p = 0.421, Q = 39.079), AFS (p = 0.636, Q = 87.686), or ANM (p = 0.603, Q = 43.858) (Table II). The “leave-one-out” analysis indicated that the findings remained consistent even with the exclusion of any individual SNP. The relatively strong findings from the MR analysis in this study suggest that AAM, AFB, AFS, and ANM are risk factors for decreased BMD.
Table II
Pleiotropy and heterogeneity tests of four female reproductive traits as genetic instrumental variables in GWAS for BMD
Discussion. Our study explored the causality between four female reproductive characteristics (AAM, ANM, AFB, and AFS) and BMD. Utilizing publicly available GWAS summary statistics from European populations, the analysis was conducted using the fixed-effects IVW method. The results indicated a negative causal relationship between AAM, AFB, and AFS with BMD, while a positive causal relationship was observed between ANM and BMD. Cochran’s Q test showed no evidence of heterogeneity, and MR-Egger regression indicated no pleiotropy. Sensitivity analysis using the leave-one-out method did not detect any SNPs that significantly affected the results, thus supporting the reliability of our findings.
This study found that AAM and an earlier ANM are linked to a higher risk of bone loss. This is in line with past research showing that later menarche and earlier menopause are associated with lower BMD [11]. The relationship between AAM and BMD may be due to fluctuating estrogen levels affecting bone formation. Estrogen is crucial for maintaining bone health, and reduced exposure to it – resulting from a later AAM – can lead to thinner, more fragile bones and an increased risk of fractures [12].
Several clinical studies on the relationship between AAM and BMD in later life have also shown that an older AAM leads to lower BMD [13]. Barron et al. [14] found that approximately 47% of bone mass in adult women is acquired within four years around menarche. Other studies suggest that between ages 16 and 20, individuals reach 60% to 80% of normal adult bone mass [15]. Therefore, an earlier AAM allows estrogen levels to reach adult levels sooner, enhancing the effects of estrogen and increasing peak bone mass. Additionally, menopause is considered a risk factor for osteoporotic fractures; the earlier the AAM and the longer the duration of menopause, the higher is the risk of OP, which is also attributed to hormone exposure [16].
Additionally, this study found a negative correlation between AFB, AFS, and BMD, consistent with previous research. The mechanisms remain unclear, but increased calcium demand during pregnancy and lactation, along with enhanced ovarian function from sexual activity, might explain the negative correlation between AFS and BMD. Research suggests that harmonious sexual activity can enhance ovarian function and hypothalamic-pituitary regulation in women, promoting estrogen secretion and potentially preventing OP. This might be a potential reason for the negative correlation between the AFS and BMD.
This study’s advantages include using MR for reliable causal inference, minimizing confounding and reverse causation, and utilizing large GWAS datasets for robust analysis, providing novel insights into the impact of female reproductive characteristics on bone health. This study is limited by its focus on European populations and use of summary statistics, which may restrict the generalizability and depth of causal insights.
In conclusion, the study found that genetic factors negatively influence BMD in relation to later AAM, AFB, and AFS, while ANM is positively associated with BMD. This suggests that sex education is crucial, as female reproductive traits can impact bone health. These reproductive characteristics should be used as indicators for predicting and preventing low BMD in postmenopausal women.