Introduction
Obstructive sleep apnea (OSA) syndrome is defined by the presence of hypopnea and apnea, which are secondary to collapse of the upper airways during sleep. OSA is seen in 2–4% of adults and according to a study conducted in Turkey the prevalence of OSA was 0.9–1.9% [1, 2]. Hypertension, hyperlipidemia, and diabetes mellitus (DM) are well-known risk factors of cardiovascular (CV) disease that cause endothelial dysfunction [3–5]. Hypoxia, hypercapnia, and fluctuations in blood pressure cause the release of vasoactive substances and impair endothelial function in patients with OSA. Endothelial function is often impaired even without obvious cardiac and vascular diseases in OSA [6, 7]. Endothelial dysfunction is associated with increased CV events [8, 9]. Endothelial dysfunction is one of the reasons for the association between CV events and OSA.
Endocan is a dermatan sulfate (a proteoglycan) and increased levels of endocan indicate endothelial dysfunction [10]. Endocan has been detected at low levels in the serum of healthy individuals, while high levels have been reported in patients with endothelial dysfunction, sepsis and psoriasis [11–13].
Few studies in the literature have investigated the relationship between endocan levels and severity of OSA [14, 15]. Furthermore, the sample size has been small in the studies that have been carried out. In the present study, we investigated the relationship between a control group and an OSA group to assess the relationship between severity of OSA and the level of endocan.
Material and methods
One hundred and seventy-nine patients without a history of cardiovascular disease who complained of snoring and were suspected to have OSA were evaluated at the outpatient clinic. All patients underwent polysomnography. They were monitored with a 58-channel polysomnograph (Sleep Screen, Compumedics, Australia) throughout the night. During the polysomnography, the following recordings were taken: 12-channel electroencephalogram (EEG), 2-channel electrooculogram (EOG), jaw and leg electromyogram (EMG), electrocardiogram (ECG), air flow via oronasal thermistor, chest and abdominal respiratory movements, oxygen saturation with a fingertip pulse oximeter, snoring with a tracheal microphone, and body posture. Sleep stages were scored based on Rechtschaffen and Kales’s standard criteria [15]. Interruptions in oronasal air flow for longer than 10 s were classified as apnea; decreases in oxygen saturation by 3% for 10 s or more or a minimum of 50% decrease in the air flow due to arousal were classified as hypopnea; and the apnea and hypopnea counts per hour were recorded as the AHI [16]. The OSAS diagnosis was made on the basis of AHI > 5. OSA patients were assigned to the mild (AHI = 5–15; n = 43), moderate (AHI = 15–30; n = 42), and severe OSA (AHI > 30; n = 55) subgroups. Sleep stages were scored following standard criteria with 30-second epochs and were reviewed and verified by a certified sleep physician.
The study was approved by the Ethics Committee in the Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Center, Training and Research Hospital. All patients signed an informed consent form. This study was performed in accordance with the requirements of the Declaration of Helsinki.
Hypertension was defined as an office blood pressure (OBP) of ≥ 140/90 mm Hg or active use of antihypertensive drugs [17]. DM was defined based on the American Diabetes Association criteria (fasting serum glucose ≥ 126 mg/dl (7 mmol/l), non-fasting glucose ≥ 200 mg/dl (11.1 mmol/l), or active use of anti-diabetic treatment) [18]. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Daily smoking habits of participations were recorded. Current smokers were defined as those who have smoked more than 100 cigarettes during their lifetime and also have smoked during the last month [19].
Hyperlipidemia was defined as fasting total serum cholesterol > 240 mg/dl, low-density lipoprotein (LDL) cholesterol > 130 mg/dl, serum triglycerides > 180 mg/dl, or the patient’s use of lipid-lowering drugs due to a history of hypercholesterolemia. Family history was defined as premature CV disease occurring in first-degree relatives aged < 55 years for men and < 65 years for women.
Blood sampling
Standard laboratory parameters, including hematocrit and creatinine levels and lipid profiles, were determined with standard methods. Blood high-sensitivity C-reactive protein (hsCRP) levels were measured using a turbidimetric method (Cobas C501 Autoanalyzer; Roche Diagnostics, Mannheim, Germany). Serum endocan levels were measured with an Aviscer Bioscience brand human endothelial cell-specific molecule 1 (ESM-1) (catalog no: SK00318-6) and through the enzyme-linked immunosorbent assay (ELISA) method using Elisa Biotek ELX800 Microplate Reader devices (intra-assay coefficient of variability (CV), 6–8%, inter-assay CV, 10–12%).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics Version 20 (IBM Corp., Armonk, NY, USA). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov) to determine whether they were normally distributed. Descriptive results are expressed as means and standard deviations (SDs) for normally distributed variables, medians and maximum-minimum for the non-normally distributed and percentages for categorical variables. Differences between continuous and categorical variables among the groups were assessed using one-way analysis of variance (ANOVA) and the Kruskal-Wallis and χ2 tests. The Mann-Whitney U or Tukey test was performed to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons. Multiple logistic regression analysis, which included endocan and others variables, was performed to identify independent predictors of OSA severity. An overall 5% type-I error level was used to infer statistical significance.
Results
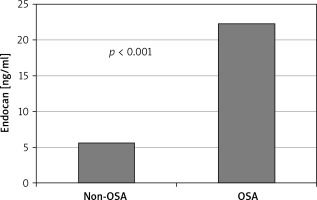
The study sample consisted of 179 patients with 140 being diagnosed as OSA and 39 non-OSA patients. Endocan levels and BMI were significantly higher in OSA patients than in the control group (11.8 (3.13–200) vs. 3.13 (3.13–23) ng/ml, p < 0.001; 32.3 ±5.0 vs. 35.9 ±5.4, p < 0.007; Table I, Figure 1).
Table I
Characteristics of the study population
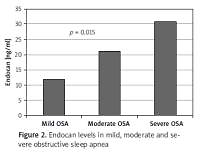
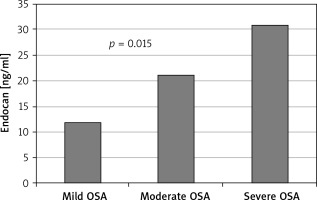
Other demographic and laboratory results were statistically similar between the groups (Table I). Levels of hsCRP and endocan were significantly higher in the severe OSA group than the mild and moderate OSA group (p = 0.008, p = 0.015, respectively; Table II). Other demographic characteristics and laboratory results were not statistically different between the groups (Table II; Figure 2).
Table II
Characteristics of the study population
Univariate logistic regression analysis showed that smoking, BMI, age and endocan levels were predictors of OSA severity. After multiple logistic regression analysis, smoking, age and endocan levels were determined to be independent predictors of OSA severity (p = 0.024, p = 0.037, p = 0.004, respectively; Table III).
Table III
Multivariate logistic regression analysis of obstructive sleep apnea severity
Discussion
In this study, endocan levels were significantly higher in patients with OSA, and the results also showed that a high level of endocan is an independent predictor of OSA severity. OSA syndrome is a substantial health problem, with a prevalence of 5–20% in the general population [20]. OSA has been determined to be an independent risk factor for CV events and mortality [21, 22]. Moreover, it was reported as a risk factor for the development of coronary artery diseases [23, 24]. Many factors observed in OSA patients, such as hypoxemia, systemic hypertension, and increased sympathetic activity, are considered to lead to atherosclerosis development [24]. Hypoxia lowers the nitric oxide level and causes increases in oxidative stress, endothelial dysfunction and LDL oxidation [25].
Endothelial dysfunction is associated with increased CV events [8, 9, 26]. It is often observed in patients with diseases associated with CV events, such as hypertension, hyperlipidemia, DM and smoking [3–5, 27]. Sleep apnea and associated factors cause a deterioration of endothelial function. Hypoxia, hypercapnia, and fluctuations in blood pressure impair endothelial function and result in the release of vasoactive substances in patients with OSA. Moreover, endothelial function is impaired in patients with OSA [6, 7]. Endothelial dysfunction is an early sign of vascular injury, and one of the causes of the development of CV disease in patients with OSA is thought to be the development of endothelial dysfunction. At the same time, the development of endothelial dysfunction has been shown to be associated with mortality and morbidity in many different diseases [8, 9, 26].
The prognostic significance of endocan level has been demonstrated in a number of diseases, such as hypertension, chronic renal disease, sepsis and transplant rejection [12, 28–30]. In the present study, it was observed that higher serum endocan levels were evident in patients with severe OSA. Also, we showed that the serum endocan level is an independent predictor of severe OSA. Recently, in a small study (40 patients), endocan levels were found to be higher in OSA patients compared with the control group [31]. The same study also showed that the serum endocan levels fall after treatment with CPAP [31]. According to this result, serum endocan levels may be a good marker for monitoring the effectiveness of treatment and severity of OSA.
In recent studies, it has been shown that the incidence of OSA increased with age, and there was a correlation between OSA and mortality and morbidity [32, 33]. It was found in several epidemiological studies that there is a significant relationship between upper airway apneas, smoking, and inflammation, and unstable sleep at night was caused by nicotine withdrawal [34, 35]. In the present study, there was an association between the severity of OSA and age and smoking.
Obesity is a major risk factor for the development of OSA. A low-calorie diet and bariatric surgery help to reduce the severity of OSA [36]. Moreover, it has been shown that an increase in weight can cause an increase in the AHI [37]. In this study, increasing BMI was significantly associated with the severity of OSA in univariate analysis (p = 0.009, p = 0.075, respectively), but there was no association in multivariate analysis.
The inflammatory process is associated with OSA [38]. hsCRP is an important marker of inflammation related to interleukin [39]. High hsCRP, tumor necrosis factor-α, interleukin-6 and interleukin-1 are increased in patients with OSA [40]. Increases in inflammation caused by hypoxia represent another cause of atherosclerosis observed along with OSA. In line with previous studies, hsCRP also was found to be higher among patients with severe OSA in the present research [40]. According to the previous studies hsCRP has been found to be associated with cardiovascular risk factors such as obesity, hyperglycemia and hypertension [41]. In our study these factors were not significantly different among OSA groups. In other OSA studies it has been shown that patients with severe OSA had coronary atherosclerosis and endothelial dysfunction more frequently [28, 42]. hsCRP is found in atheromatous plaque and it is associated with vascular dysfunction. Increased hsCRP levels in patients with severe OSA may be an indicator of endothelial dysfunction and coronary atherosclerosis.
Our study has several limitations. First, it was a non-randomized study, and therefore subject to selection bias. Moreover, we could not assess endothelial dysfunction by flow-mediated dilatation (FMD) and other validated techniques. However, in previous studies serum endocan levels were shown to be independently correlated with FMD [28, 31]. Second, CPAP, metabolic syndrome and pharmacological therapy can alter endothelial function [31, 43]. In this study the relationships between CPAP, metabolic syndrome and pharmacological treatment and endocan levels were not investigated. Finally, the long-term results were not presented in this article.
In conclusion, high levels of endocan were associated with the severity of OSA. Endocan may be useful for risk classification in this patient group.