Introduction
At present, the incidence of lung cancer ranks first among malignant tumors, the age of peak onset is within 60–79 years old, and thoracic surgery remains the main treatment for early lung cancer [1].
In the anesthesia for thoracic surgery, one-lung ventilation (OLV) is a very important method of ventilation. It can well maintain the ventilation for patients and prevent this from affecting the operative procedure [2]. However, OLV is likely to cause lung injury and hypoxemia [3]. Although the incidence of moderate to severe postoperative pulmonary complications (PPCs) after thoracic surgery is not known accurately, it ranges between 15% and 32% in studies with large sample sizes [4–6].
The mechanism of OLV causing lung injury [7] is divided into four types: pressure injury, volume trauma, injury related to collapse of alveoli and the biological injury caused by the above lung injuries. It has been reported [8] that the release of inflammatory cytokines from the alveolar surface can be triggered by mechanical ventilation for a few minutes, which further leads to local inflammatory response of the lung. Collapse of the lung on the operated side will cause ischemic/hypoxic injury of this side of the lung. When ventilation is resumed for the two lungs, there will be an increase in the blood in the pulmonary vascular bed on the operated side. As a result, free oxygen radicals and some harmful cytokines will be produced, which causes ischemia-reperfusion injury of the lung on the operated side [9].
Biological injury of the lung as one mechanism of lung injury plays a very important role, and so does the oxidative stress response [10]. Also, OLV-related hypoxemia is closely correlated to intraoperative intrapulmonary shunt fraction (Qs/Qt) [10]. During OLV, reducing lung injury and Qs/Qt caused by mechanical ventilation is highly necessary.
With age, the immune function of the elderly decreases, and the physiological functions of the organs gradually attenuate. In elderly patients, the physiology of respiratory and circulatory systems significantly changes during anesthesia with OLV for thoracotomy [7, 11, 12]. In elderly patients, the incidence of perioperative complications during thoracotomy significantly increases due to the attenuation of physiological function. Elderly patients undergoing thoracotomy are more likely to develop pulmonary complications during the perioperative period. Shiono et al. reported that [10, 13] 5.1–6.1% of elderly patients develop pulmonary infection after thoracotomy. Advanced age is already identified as an independent risk factor of lung injury caused by mechanical ventilation [14].
Dexmedetomidine (DEX) is a highly selective α2 adrenergic receptor agonist. It has effects of analgesia, anti-anxiety, anti-sympathetic, inhibition of saliva secretion, inhibition of the stress response and hemodynamic stability, and can reduce the occurrence of tissue injury by inhibiting inflammatory reactions [15]. There are a large number of α2-receptors on the bronchial mucosa. It has been shown [10] that DEX acts on the α2-receptors, inhibits the inflammatory response, and relieves the ischemia/reperfusion injury, thereby exerting a protective effect on the organs [16, 17].
However, the use of DEX in elderly patients receiving OLV during anesthesia has not been reported. The physiological mechanism is unique in elderly patients receiving OLV and these patients have a higher risk of perioperative complications [13]. This study investigated the use of DEX in elderly patients receiving OLV during thoracotomy. The authors hypothesized that DEX may reduce lung injury and reduce respiratory as well as cardiovascular complications in the perioperative period in elderly patients.
Material and methods
Data and methods
This study was approved by the Ethics Committee of our hospital, and all patients provided signed informed consent.
The patients were recruited from Beijing Chest Hospital affiliated to Capital Medical University from October 2015 to December 2018. Inclusion criteria: (1) aged 60–86 years old; (2) grade II or III according to American Society of Anesthesiologists (ASA) physical status classification system; (3) no history of long-term heavy smoking; (4) no apparent abnormalities of heart, liver and kidney functions. Exclusion criteria: (1) preoperative long-term history of hormone therapy; (2) preoperative arterial oxygen saturation (SpO2) below 90%; (3) difficult airway; (4) already combined with pulmonary insufficiency, such as acute upper respiratory tract diseases, chronic obstructive pulmonary disease (COPD) or asthma; (5) intraoperative blood transfusions exceeding 400 ml.
These patients were randomly divided into two groups (n = 60) using the random number table method: the DEX group (group D), and the control group (group C). At 30 min before anesthesia, patients were intramuscularly injected with 1 mg of penehyclidine hydrochloride. After the patient was transferred to the operation room, the vein access was opened, and the electrocardiogram (ECG), SpO2, mean arterial pressure (MAP) and bispectral index (BIS) were routinely monitored. At 15 min before anesthesia induction, patients in group D were intravenously injected with DEX at a rate of 0.5 μg/kg/h until sternal closure. Patients in group C were intravenously injected with equal volumes of normal saline.
Anesthesia induction: Patients were successively and intravenously injected with 0.4 μg/kg of sufentanil and 0.04 mg/kg of midazolam. Then, they received a target-controlled infusion (TCI) of propofol (target plasma concentration: 2.5–3.0 μg/ml) and 0.2 mg/kg of cisatracurium, and dual-lumen bronchial intubation was performed. Subsequently, the fiberbronchoscope was located, and mechanical ventilation was performed. Parameters for two-lung ventilation: tidal volume, 8–10 ml/kg (based on standard weight); frequency of respiration, 10–14/min; inspiratory-to-expiratory ratio, 1.0 : 1.5. Parameters for OLV: tidal volume, 7 ml/kg; frequency of respiration, 12–16/min; inspiratory-to-expiratory ratio, 1 : 1.5. Target-controlled infusion of remifentanil hydrochloride (target plasma concentration: 2.5–3.0 ng/ml) was performed.
Anesthetic maintenance: TCI of propofol was performed intraoperatively to achieve the target plasma concentration of 2~3.5 μg/kg. Remifentanil hydrochloride (target plasma concentration 2.5~3 ng/ml) was injected through TCI, and intravenous injection of cisatracurium besylate (5 mg) was performed if necessary. Bispectral index was maintained within 40–60, heart rate (HR) within 60–100 beats/min, and MAP fluctuation not above 20% of the baseline. If MAP was higher by over 20% of the baseline for more than 1 min and the influence of anesthetic depth was excluded, 12.5 mg of urapidil was given through intravenous injection; if MAP was lower by over 20% of the baseline for more than 1 min and infusion of fluid (50 ml) within 5–10 min took no effect, then after excluding the influence of anesthetic depth, 6 mg of ephedrine was intravenously injected; if HR ≤ 50 beats/min or ≥ 100 beats/min, then atropine (0.2 mg) and esmolol (5 mg) were intravenously injected, respectively. All these vasoactive drugs could be repeatedly used. If these measures still failed to stabilize the circulation, the patients were excluded from the experiment. A bypass collateral circulation monitoring system was used. Peak airway pressure (Ppeak), platform pressure (Pplat) and dynamic pulmonary compliance (Cdyn) were recorded every 5 min.
After surgery, the patients were given patient-controlled intravenous analgesia (PCIA) using sufentanil (4 μg/kg) and tropisetron (10 mg), which was diluted with normal saline to 100 ml. The background dose was 2 ml/h, and the patient-controlled dose was 1 ml each time, with a lockout period of 15 min.
The primary indicators were Pplat, Ppeak, Cdyn and arterial partial pressure of oxygen (PaO2). Pplat, Ppeak, Cdyn, stroke volume variability (SVV), cardiac index (CI), central venous pressure (CVP) and MAP were measured at 5 min before OLV in a lateral position (T1), 30 after OLV (T2) and 10 min after two-lung ventilation (T3); blood gas analysis was performed for the arterial and venous blood samples collected at T1, T2, T3 and 2 h after surgery (T4), PaO2 and arterial partial pressure of carbon dioxide (PaCO2) were recorded, and Qs/Qt was calculated.
Secondary indicators: Venous blood was sampled at T1 and 24 h after surgery (T7). The concentrations of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were detected for each group using enzyme-linked immunosorbent assay (ELISA). The pain scores at T4 and 6 h after surgery (T6) were assessed. Visual Analogue Scale (VAS) score and Ramsay Sedation Scale score at T5 (4 h after surgery) were also assessed.
Respiratory and circulation complications were observed after surgery: pulmonary infection, pyothorax, atelectasis, pleural effusion, postoperative hypoxemia, arrhythmia and myocardial infarction.
Statistical analysis
Data were analyzed using the statistical software SPSS 17.0. Measurement data were expressed as mean ± standard deviation (x ± SD). If the variance was homogeneous, inter-group comparison was conducted using a group t-test. If the variance was not homogeneous, inter-group comparison was conducted using the rank sum test. Intra-group comparison was conducted using repeated-measures analysis of variance. Count data were compared using Fisher’s exact test and p < 0.05 was considered statistically significant. Counting data were compared using the χ2 test and p < 0.05 was considered statistically significant.
Results
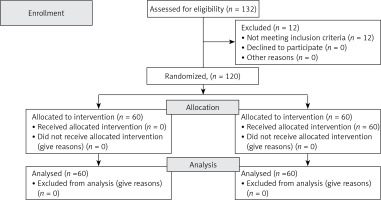
(1) One hundred and thirty-two elderly patients with lung cancer were recruited. After excluding 12 patients, 120 patients were eligible (Figure 1).
There were no significant differences in gender, age, body mass index (BMI), ASA grading, and pulmonary function between the two groups (p > 0.05). Nor was there a significant difference in anesthesia duration, duration of surgery, duration of OLV, or intraoperative urine volume (p > 0.05) (Table I).
Table I
General data of patients in the two groups and their conditions during the operation (n = 60 in each group)
(2) Changes in respiratory mechanics and hemodynamic indicators in the two groups (Table II).
Table II
Changes of respiratory mechanics and hemodynamics at different time points for the patients in both group (x ± s)
At T2, compared with T1, there was a significant decrease in SVV and CI in the two groups (p < 0.05). At T2 and T3, compared with group C, group D had a significant decrease in CI, MAP and CVP (p < 0.05). At T2, compared with group C, group D had a significant increase in SVV (p < 0.05). At T2, compared with group C, group D had a significant decrease in SVV (p < 0.05). There was no significant difference in intraoperative Ppeak and Pplat between the two groups (p > 0.05).
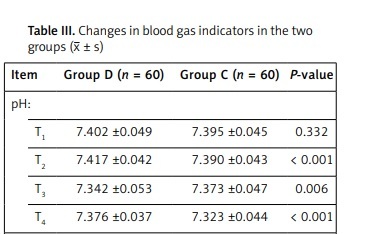
(3) Changes in blood gas indicators in the two groups (Table III).
Table III
Changes in blood gas indicators in the two groups (x ± s)
At T2, T3 and T4, compared with group C, group D had a significant increase in pH and PaO2 (p < 0.05). At T2, T3 and T4, compared with group C, group D had a significant decrease in Qs/Qt (p < 0.05).
(4) Changes in TNF-α and IL-6 concentrations in the two groups (Table IV).
Table IV
Changes in tumor necrosis factor-α (TNF-α) and interleukine-6 (IL-6) concentrations in the two groups (pg/ml, x ± s)
| Item | Group D (n = 60) | Group C (n = 60) | P-value |
|---|---|---|---|
| TNF-α: | |||
| T1 | 9.3 ±3.5 | 9.7 ±3.4 | 0.479 |
| T6 | 14.9 ±1.8 | 15.8 ±2.8 | 0.048 |
| IL-6: | |||
| T1 | 15.6 ±2.6 | 15.3 ±1.9 | 0.420 |
| T6 | 15.9 ±2.7 | 16.8 ±2.4 | 0.045 |
At T6, compared with group C, group D had a significant decrease in the venous concentrations of TNF-α and IL-6 (p < 0.05).
(5) VAS and Ramsay Sedation Scale scores of the two groups (Table V).
Table V
Visual Analogue Scale (VAS) and Ramsay Sedation Scale scores of the two groups (x ± s)
| Item | Group D (n = 60) | Group C (n = 60) | P-value |
|---|---|---|---|
| VAS: | |||
| T4 | 3.2 ±1.7 | 4.7 ±1.6 | < 0.001 |
| T5 | 4.5 ±1.3 | 4.9 ±1.2 | 0.025 |
| Ramsay Sedation Scale: | |||
| T4 | 2.6 ±0.9 | 1.5 ±0.6 | < 0.001 |
| T5 | 1.6 ±0.6 | 1.4 ±0.5 | 0.016 |
At T4 and T5, compared with group C, group D had a significant decrease in VAS score (p < 0.05), and significant increase in Ramsay Sedation Scale score (p < 0.05).
(6) Perioperative complications in the two groups (Table VI).
Table VI
Perioperative complications in the two groups
Compared with group C, the occurrence of respiratory and cardiovascular complications decreased significantly in group D (p < 0.05).
(7) Doses of each anesthetic used in the two groups (Table VII).
Table VII
Doses of anesthetics used in the two groups (x ± s)
Compared with group C, the doses of propofol, cisatracurium and sufentanil decreased significantly in group D (p < 0.05), while the use of vasoactive agents increased (p < 0.05).
Discussion
Dexmedetomidine can decrease Qs/Qt and increase PaO2 during OLV and 2 h after surgery. Dexmedetomidine can decrease the bronchoalveolar lavage fluid (BALF) concentrations of TNF-α and IL-6 at 24 h after surgery in patients undergoing thoracotomy, thus further relieving lung injury. Dexmedetomidine can decrease pain scores at 2 h and 6 h after surgery. Dexmedetomidine also significantly reduced the intraoperative doses of propofol, sufentanil and cisatracurium. However, DEX caused a reduction in CI, CVP and MAP, which led to much higher doses of vasoactive agents during surgery. This deserves extra attention from anesthesiologists.
Qs/Qt remained low during OLV and at 2 h after surgery in patients receiving DEX. The reasons may be as follows: (1) DEX relieves oxidative stress response of the lungs [18], thereby reducing lung injury caused by oxidative stress. Thus, better gas exchange in the lung is ensured after surgery; (2) it has been reported in the literature [10] that DEX could reduce Qs/Qt, thereby improving OI; (3) DEX has an analgesic effect [19], which is conducive to gas exchange of the lungs and to the prevention of hypoxemia; (4) DEX can reduce the dose of muscle relaxants, thereby reducing the residue of muscle relaxants after surgery. It has been reported in the literature [20] that the residue of muscle relaxants after surgery is great, which is correlated with postoperative complications. Therefore, the minimal residue of muscle relaxants after surgery can help achieve easier respiration and reduce the occurrence of hypoxemia postoperatively.
Neutrophils, alveolar macrophages and alveolar epithelial cells will produce a large number of inflammatory mediators after mechanical injury [21]. As a result, tissue injury will occur, as well as a large number of inflammatory mediators and inflammatory cells. Interleukin-6 is an important indicator of severity of early tissue injury [22]. Tumor necrosis factor-α is an important sensitive indicator of tissue injury [23]. Tumor necrosis factor-α and IL-6 are both important sensitive indexes to reflect the severity of lung tissue injury.
The BALF levels of TNF-α and IL-6 can be used to assess pulmonary infection [24]. There have been many reports on TNF-α and IL-6 in BALF as indicators for pulmonary inflammatory diseases [25, 26].
When mechanical injury occurs, neutrophils, alveolar macrophages and alveolar epithelial cells can produce a large number of inflammatory mediators, causing lung tissue damage.
If there is a significant increase in the BALF levels of IL-6 and TNF-α after surgery, this usually indicates lung injury caused by thoracotomy and mechanical ventilation. However, DEX can inhibit the production of inflammatory factors, thereby relieving lung injury.
In our study, the pain scores decreased significantly at 2 and 6 h after surgery. Sufficient analgesia is of particular importance in patients receiving thoracic surgery. As reported in the literature [27], postoperative hypoxemia and cardiovascular complications were closely correlated with insufficient postoperative analgesia, and proper analgesia could obviously reduce such occurrence. Due to the analgesic effect of DEX, the patients can breathe more easily after surgery, which helps reduce hypoxemia and CO2 accumulation and further improves the postoperative internal environment. Stability of the internal environment can reduce perioperative cardiovascular events.
The use of DEX could significantly reduce the intraoperative doses of propofol, cisatracurium and sufentanil. Propofol can increase intrapulmonary shunt, as was found in the DEX group in the present study. This helps reduce the postoperative residue of anesthetics. It has been reported in the literature [21] that the residue rate of muscle relaxants was up to 3.5–88% after surgery and that the postoperative complications were correlated with this. An overdose of opioids will inhibit respiration. In our study, the use of DEX significantly reduced the doses of muscle relaxants and opioids, which further reduced postoperative residues of muscle relaxants and opioids. Therefore, the patients were able to breathe more easily and had better gas exchange in the lungs.
Moreover, DEX stabilizes respiratory mechanics and hemodynamics, though the intraoperative incidence of sinus bradycardia increases considerably with a much higher use of intraoperative vasoactive agents. Dexmedetomidine is an α-adrenergic receptor agonist, which agrees with the reported ability of DEX in reducing BP and HP [18]. This fact deserves extra attention from anesthesiologists.
The present study had the following limitations. First, a high fraction of inspiration O2 (FIO2) can possibly increase the risk of pulmonary atelectasis, lead to absent ventilation in low Qs/Qt of the lung and result in a large alveolar-arterial O2 tension difference [28]. Second, surgical trauma will also affect the lungs, leading to the generation of pro-inflammatory cytokines. Third, lung cancer patients already tend to have a higher level of cytokines before surgery, which will inevitably influence the results. Fourth, this is not a double-blinded study, and the anesthetist knew well about all patients. Thus, bias cannot be avoided.
In conclusion, in elderly patients, DEX can reduce Qs/Qt and improve the oxygenation index (OI) and reduce lung injury caused by OLV. Moreover, DEX reduces the perioperative cardiovascular complications, as well as the intraoperative doses of propofol, cisatracurium and sufentanil. The use of DEX can offer a certain protective effect for the lungs in elderly patients receiving thoracic surgery. However, the findings of the present study remain to be further validated.