Introduction
Cervical cancer is one of the cancers in the female population [1]. The prevalence of cervical cancer has been rising in recent years [2] with unknown pathogenesis [3]. Because of the anatomical features, early diagnosis of female genital cancer is not common; most patients are at advanced stages at the time of diagnosis, although cervical cancer is easier to diagnose than ovarian cancer and endometrial cancer [4]. The treatment of cervical cancer is not satisfactory currently [5]. Therefore, it is important to invent novel therapies for cervical cancer.
Survivin is a protein encoded by the BIRC5 gene in humans; it is also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5 [6]. Survivin belongs to the apoptosis inhibitor family; it specifically inhibits caspase activation, which results in negative regulation of apoptosis [7]. Published data indicate that the breakdown of survivin expression can inhibit cancer growth [8]. It is also reported that most cancer cells express survivin while most terminal body cells do not [9]. This fact implies that survivin is an optimal potential target of cancer treatment.
Upon activation, regulatory B cells produce interleukin (IL)-10 to suppress other effector immune cell activities, such as to inhibit cytotoxic CD8+ T cell functions; thus, they are also called B10 cells [10]. Similar to the regulatory T cells, B10 cells are beneficial to the tumor escaping from immune surveillance by interfering with the anti-tumor immunity [11]. On the other hand, B10 cells also inhibit apoptosis, which may contribute to cancer development [12]. Published data indicate that survivin and IL-10 increase in cancer-bearing mice [13]. Thus, we hypothesize that survivin may up-regulate the expression of IL-10 in B cells. In this study, we found that the levels of survivin were higher in cervical cancer than normal tissue. A positive correlation was identified between survivin and IL-10 mRNA in intratumoral B cells. Administration of survivin antisense efficiently inhibited cervical cancer growth and decreased the frequency of B10 cells in the cervical cancer tissue in a mouse model.
Material and methods
Patients
Ten patients with cervical cancer were recruited to this study. The diagnosis and treatment of cervical cancer were performed by doctors in our department following routine procedures. Patients treated with immune suppressive agents or radiotherapy or with autoimmune diseases were excluded from the study. The demographic data of the patients are presented in Table I. The experimental procedures were approved by the Human Ethics Committee at Shandong University. Informed written consent was obtained from each patient.
Preparation of single cells with surgically removed cervical cancer
Cervical cancer tissue was collected from the operation room immediately after removal from the patients. The tissue was cut into small pieces (about 2 × 2 × 2 mm) and incubated with 0.5 mg/ml collagen IV (Sigma Aldrich) for 2 h at 37°C with mild agitation. The cells were harvested by filtering through a cell strainer (40 μm) and centrifugation.
Isolation of B cells
By magnetic cell sorting (MACS) with commercial reagent kits (Miltenyi Biotech) following the manufacturer’s instructions, CD19+ B cells were isolated from the single cell preparation of cervical cancer as described above, or from the peripheral blood mononuclear cells collected from healthy subjects. The B cell purity was 99% as checked by flow cytometry.
Cell culture
B cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.5 mg/ml streptomycin, 2 mM L-glutamine and 20 ng/ml anti-CD40 mAb. The medium was changed in 2–3 days. The viability of the cells was greater than 99% as checked by the trypan blue exclusion assay before being used for further experiments.
Real time quantitative RT-PCR (RT-qPCR)
Total RNAs were extracted from B cells or cervical cancer tissue with TRYzol reagents (Invitrogen). The cDNA was synthesized with the RNA samples and a reverse transcription kit (Invitrogen). The samples were then amplified on a real-time PCR device (CFX96 Touch; Bio Rad) with the SYBR Green Master Mix (Invitrogen). The primers used in the present study include tgacccatggactgaacaca and acagtcacagagagtccagc (survivin), and gttctttggggagccaacag and gctccctggtttctcttcct (IL-10). The results were calculated with the 2–ΔΔCt method and are presented as fold change compared to the control group.
Western blotting
The total proteins were extracted from the cervical cancer tissue. The proteins were fractioned with SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred onto a PVDF membrane. After blocking with 5% skim milk for 30 min at room temperature, the membrane was incubated with anti-survivin mAb (1 : 200; Santa Cruz Biotech) overnight. The membrane was then washed with TBST (Tris-buffered saline Tween 20) 3 times with shaking, followed by incubating with peroxidase-labeled second antibodies (Santa Cruz Biotech) for 1 h at room temperature and washed again with TBST 3 times. The immune complex on the membrane was developed with ECL (enhanced chemiluminescence; GE Healthcare). The results were photographed with an image system (UVI, Cambridge, UK).
Assessment of the capacity of survivin for regulation of IL-10 expression in B cells
CD19+ B cells were isolated from the peripheral blood samples collected from healthy controls. B cells were cultured (106 cells/ml; each sample was tested in triplicate) in the presence of recombinant survivin (R&D Systems) at gradient concentrations for 48 h. The B cells were then harvested to be analyzed by RT-qPCR to determine the IL-10 mRNA levels.
Preparation of antisense of survivin-carrying liposomes
The antisense of survivin was designed, synthesized and constructed into liposomes by Enke Biotech (Shenzhen, China). The sequences of the antisense tested by Enke Biotech are listed in Table II, from which the No. 2 sequence was used in the study.
Preparation of cervical cancer cell line
U14 cells, a cell line of mouse cervical cancer, were obtained from the Cell Bank at Union Medical University (Beijing, China). The cells were cultured in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.5 mg/ml streptomycin and 2 mM L-glutamine. The medium was changed every day. The cell viability was greater than 99% as checked by the trypan blue exclusion assay before use in further experiments.
Assessment of the effects of antisense of survivin on suppression of survivin expression in cervical cancer cells
U14 cells (106 cells/ml) were treated with the antisense of survivin-carrying liposomes (provided by Enke Biotech, Shenzhen, China) in the culture at 1 μg/ml (suggested by the manufacturer) for 48 h. The cells were then harvested and analyzed by RT-qPCR to determine the levels of survivin in the cells. Each sample was tested in triplicate. Each experiment was repeated 3 times.
Mice
Female C57B6 mice were purchased from the Shanghai Xinmao Experimental Animal Center (Shanghai, China). The mice were maintained in a pathogen-free environment and allowed to access food and water freely. The experimental procedures were approved by the Animal Care Committee at Shandong University (approval number: SDU20150023).
Preparation of cervical cancer-bearing mice and treatment with antisense of survivin
The mice were subcutaneously injected with U14 cells (106 cells/mouse) at the back skin. The mice were grouped (n = 10) into the saline group (treated with saline), antisense group (treated with antisense of survivin-liposomes, 0.5 mg/mouse, daily intraperitoneal injection from day 7 to day 12) and the control group (treated with empty liposomes). The tumor size (4/3 × 3.14 × (a × b × c*)) in each mouse was recorded daily. (*a, b and c are the half size of height, width and length of the tumor).
Assessment of the frequency of B10 cells in experimental cervical cancer tissue
After sacrifice, the tumor mass was removed from each mouse. Single cells were prepared with the tumor tissue as described above. The cells were stained with FITC-labeled anti-CD19 antibody (or isotype IgG) for 30 min at 4°C. After washing with phosphate-buffered saline (PBS), the cells were fixed with 1% paraformaldehyde for 1 h at room temperature, followed by incubation with 0.5% saponin for 30 min. The cells were then incubated with APC-labeled anti-IL-10 antibody (or isotype IgG) for 30 min at 4°C. After washing with PBS, the cells were analyzed with a flow cytometer (FACSCanto II, BD Bioscience). The data were analyzed with Flowjo (TreeStar) with the isotype IgG staining data as a gating reference.
Results
High levels of survivin are detected in cervical cancer
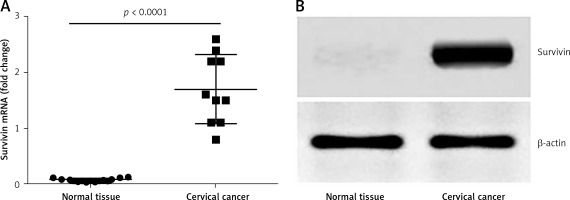
To investigate the role of survivin in the pathogenesis of cervical cancer, we collected 10 samples from surgically removed cervical cancer in the operation room. The samples were analyzed by RT-qPCR and Western blotting. The results showed that the levels of survivin were significantly higher in cancer tissue than those in normal tissue (Figure 1). The results indicate that survivin may be associated with the development of cervical cancer.
Figure 1
Survivin levels in cervical cancer tissue. A – The scatter dot plots show the survivin mRNA levels in cervical cancer tissue and the marginal normal tissue of patients with cervical cancer (n = 10). B – The representative immune blots show the protein levels of survivin in cervical cancer tissue and normal tissue from 10 patients with cervical cancer
IL-10 levels in intra-cervical cancer B cells are correlated with survivin levels in cervical cancer
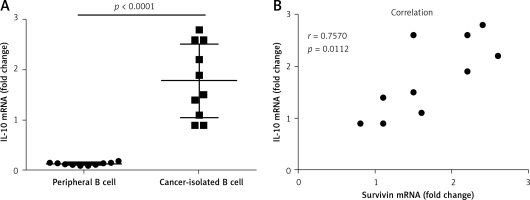
To elucidate the relation between survivin and the expression of IL-10 in B cells, we measured the levels of IL-10 mRNA in B cells isolated from cervical cancer tissue. The results showed that the levels of IL-10 mRNA in the cancer tissue-isolated B cells were higher than those in peripheral B cells (Figure 2 A). A correlation assay was performed with the data of IL-10 and survivin. The results showed a positive correlation between the IL-10 mRNA levels and the survivin levels in the cervical tissue (Figure 2 B). The results suggest that survivin may play a role in up-regulating IL-10 expression in B cells.
Figure 2
Assessment of the correlation between survivin in cervical cancer and IL-10 in intratumoral B cells. A – The scatter dot plots show the IL-10 mRNA levels in the intratumoral B cells and the peripheral B cells. B – The scatter dot plots show the correlation between survivin in cervical cancer tissue and the IL-10 mRNA levels in the intratumoral B cells
Survivin increases IL-10 expression in B cells
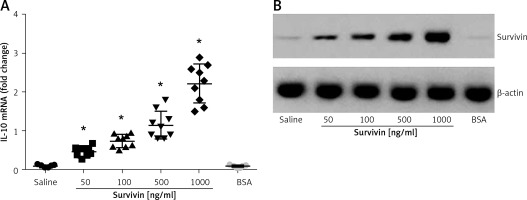
The data reported above suggest that survivin may up-regulate the expression of IL-10 in B cells. To test this, we isolated B cells from healthy persons. The B cells were cultured in the presence of survivin for 48 h. As analyzed by RT-qPCR and Western blotting, the presence of survivin in the culture markedly increased the expression of IL-10 in B cells at both mRNA and protein levels in a survivin dose-dependent manner (Figure 3). The results demonstrate that survivin does increase the expression of IL-10 in B cells.
Figure 3
Survivin increases the expression of IL-10 in B cells. A – The scatter dot plots show the IL-10 mRNA levels in B cells after culturing in the presence of survivin (as denoted on the X axis) for 48 h. Each dot represents an individual datum. *p < 0.0001, compared with the saline group. BSA: bovine serum albumin; used as a control agent (1000 ng/ml). B – The immune blots indicate the protein levels of survivin. The data were from 3 independent experiments; three samples were tested in triplicate in each experiment
Antisense of survivin inhibits cervical cancer growth in mice
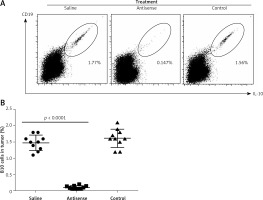
The data presented in Figures 1–3 suggest that survivin may contribute to cervical cancer growth. To test this, we designed antisense of survivin. As tested by the cell culture study, the designed antisense sequences showed suppressor effects on survivin expression in U14 cells (a mouse cervical cancer cell line) (Figure 4). We then developed a mouse model of cervical cancer with U14 cell transplantation. The cancer-bearing mice were treated with the survivin antisense-carrying liposomes daily for 9 days. From the tumor size records, we found that administration of survivin antisense efficiently inhibited the cervical cancer growth (Figure 5). We also assessed the frequency of B10 cells in the tumor. The results showed that administration of survivin antisense significantly decreased the frequency of B10 cells in the tumor tissue (Figure 6).
Figure 4
Effects of antisense on survivin expression in cervical cancer cells. The scatter dot plots show the survivin mRNA levels in U14 cells after treatment with saline or antisense of survivin-carrying liposomes. The data were from 3 independent experiments; three samples were tested in triplicate in each experiment
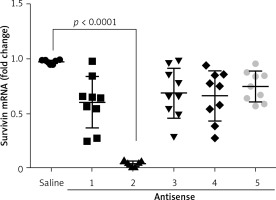
Figure 5
Records of tumor size. The curves indicate the tumor size in mice during the period of treatment as denoted in the figure. Each group consists of 10 mice
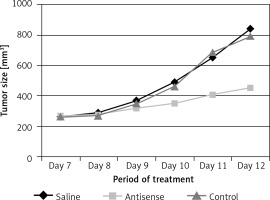
Figure 6
B10 cells in experimental cervical cancer. A – The flow cytometry dot plots show the frequency of B10 cells in the cervical cancer tissue. B – The scatter dot plots show the individual data of B10 cells in the cervical cancer tissue of each tumor- bearing mouse. Each group consists of 10 mice
Discussion
The therapeutic efficacy of cervical cancer is unsatisfactory currently [14, 15]. The optimal treatment time and radiation dose are critical to the therapeutic efficacy and are significantly associated with survival improvement [14]. Thus, it is important to invent novel therapeutic remedies for cervical cancer. The present study aims to assess the effects of antisense of survivin on cervical cancer. From the cervical cancer tissue which we collected from patients with cervical cancer tissue we found high levels of survivin in cervical cancer. We also found high frequency of B10 cells in cervical cancer. Because a positive correlation was identified between the frequency of B10 cells and the survivin levels in cervical cancer, we treated B cells collected from healthy persons with recombinant survivin in the culture. The results showed that exogenous survivin markedly increased the expression of IL-10 in B cells. By treating tumor-bearing mice with antisense of survivin, we found that the antisense of survivin efficiently inhibited cervical cancer growth.
If the cervical cancer is diagnosed at the early stage without metastasis, treatment with surgery to radically remove the cancer can achieve good results [4]. However, if the cervical cancer is found at the advanced stage, especially those with remote metastasis, treatment with surgery alone may be unsatisfactory [4]; some adjuvant remedies, such as radiotherapy, chemotherapy and immunotherapy, may be employed before and/or after surgery [16]. The present data show that gene therapy inhibiting survivin may be an additional remedy for the treatment of cervical cancer. Although using the antisense of survivin did not completely remove the experimental cervical cancer from mice, it significantly inhibited the cancer growth in mice. Therefore, this method has the potential to be a supportive method in the treatment of cervical cancer.
In this study, we identified a positive correlation between survivin and B10 cells in cervical cancer tissue collected from patients with cervical cancer. B10 cells are a fraction of immune regulatory cells. Cumulative reports indicate that immune regulatory cells, including regulatory T cells and regulatory B cells, play a critical role in the pathogenesis of cancer [17, 18]. The underlying mechanism is that the immune regulatory cells are capable of suppressing the antitumor immunity, such as inhibiting CD8+ cytotoxic cells [19, 20].
The present data show that the B cells isolated from cervical cancer tissue had high levels of IL-10 expression, which was correlated with the levels of survivin in the same cancer tissue. We understood that this phenomenon could be a coincidence or a causal relationship between survivin and IL-10 expression in B cells. With an in vitro cell culture study, we were able to demonstrate that survivin was capable of up-regulating the IL-10 expression in B cells. The results suggest that cervical cancer cells produce survivin; the latter increases IL-10 expression in the intratumoral B cells to confer the B cells with immune suppressor function, which compromises the antitumor immunity in the local tissue. Thus, we designed an antisense of survivin carried by liposomes. The administration of the antisense-carrying liposome efficiently inhibited the experimental cervical cancer growth. Although the antisense could not radically remove the tumor, the tumor mass was significantly smaller than the control tumor mass.
In conclusion, the present study revealed that cervical cancer expressed high levels of survivin, which was correlated with the expression of IL-10 in the intratumoral B cells. Administration of antisense of survivin efficiently inhibited the experimental cervical cancer growth and decreased the frequency of B10 cells in the cervical cancer. The data suggest that the antisense of survivin may be a potential adjuvant therapeutic remedy for cervical cancer.


