Hyperlipidaemia is considered to be an abnormal change of total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol in blood lipids. Hyperlipidaemia also causes atherosclerosis, which is a risk factor for cardiovascular and cerebral vascular diseases, raises the morbidity rate of pancreatitis, and increases the risk of tumours [1]. The incidence of hyperlipidaemia, a disorder of lipid metabolism, is increasing year by year with the improvement of people’s living standards, and changes in lifestyles and dietary habits, and there is a tendency for the disease to develop at a younger age, i.e. the detection rate of hyperlipidaemia in children and adolescents is increasing, which poses a great challenge to public health.
The effect of exercise on reducing the risk of hyperlipidaemia has been observed in a number of observational studies and randomised controlled trials, but no association between exercise and changes in the risk of hyperlipidaemia has been reported in cross-sectional studies [2], and the contradictory results may be due to potential confounders, and the relationship between “exposure” and “outcome” may be unclear. In addition, there is a possibility of reverse causality between “exposure” and “outcome”, and Mendelian randomisation, a genetic method increasingly used to infer causality, is used to infer causality by calculating the Wald value of single nucleotide polymorphisms for outcome and the SNP effect for exposure [3].
We therefore used 2-way 2-sample Mendelian randomisation to infer causality between exercise and hyperlipidaemia by analysing a legacy genome-wide association study (GWAS) in a European population sample.
Methods
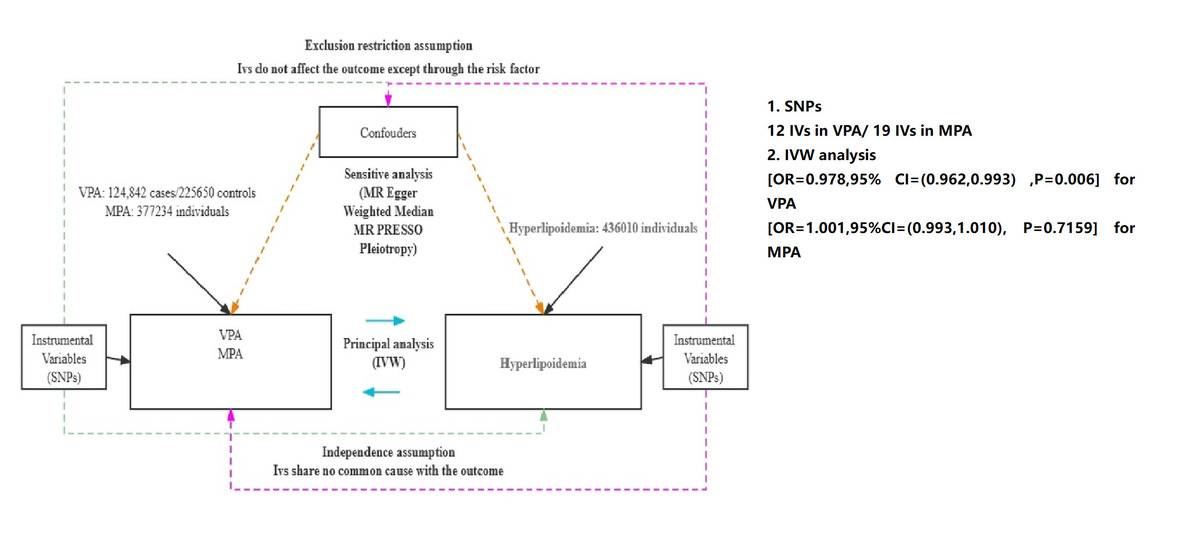
This study used bidirectional 2-sample Mendelian randomisation, whereby exposures and outcomes were taken from separate databases to reduce potential heterogeneity interference. The 2-way analysis was performed by reversing the order of exposure and outcome and then Mendelian randomisation once again to examine whether there was reverse causality between exposure and outcome. The outcome, exposure, and instrumental variables were obtained from published GWAS meta-analyses, and the populations were all from Europe. GWAS data on exercise were obtained from the EBI database, and data on hyperlipidaemia were obtained from the UK Biobank, and their relevant information can be found on the open GWAS website (IEU OpenGWAS project (mrcieu.ac.uk)). A total of 2 types of exercise were chosen for our study: moderate physical activity (MPA) and moderate-high intensity physical activity (VPA). For MPA the overview was that participants performed low-intensity activities daily, such as cycling at normal speed, weighted walking, etc. For VPA the overview is that participants perform moderate- to high-intensity activities such as fast bike riding, aerobic exercise, or moderate resistance training with a heart rate of approximately 55–85% of maximum heart rate on a daily basis.
Results
Firstly, a total of 12 inverse variances (IVs) were found to be associated with VPA (excluding 2 return SNPs), and 19 IVs were found to be associated with MPA (excluding one return SNP), and the F statistic values were all greater than 10, which was considered to indicate no weak instrumental bias, and such IVs fulfilled the first hypothesis of MR, i.e. the IVs are closely associated with exposure.
Secondly, in IVW analysis, a negative causal relationship was found between VPA and the risk of hyperlipidaemia (OR = 0.978, 95% CI = (0.962, 0.993); p = 0.006), and the results of weighted median also showed a negative causal relationship between them (OR = 0.979, 95% CI = (0.959, 0.999); p = 0.0458); for MPA, no causal relationship was found with hyperlipidaemia (OR = 1.001, 95% CI = (0.993, 1.010); p = 0.7159). To verify the existence of possible reverse causality between the two, we also performed a reverse Mendelian randomisation analysis, which showed that there was no reverse causality between hyperlipidaemia and VPA (PVPA = 0.1804), and no reverse causality with MPA (PMPA = 0.2241), which suggests that our study was not influenced by reverse causality.
Again, to further validate the reliability of IVW, we tested the heterogeneity of the MR results, and there was no heterogeneity in all the analyses (p > 0.05). In addition to this, to exclude the horizontal polytomousness, which has a key influence on the results, we also tested it, and there was no horizontal polytomousness in all the results, which indicates that the analyses of MR are robust.
Finally, to further verify the reliability of the IVW results, we performed a leave-one-out cross-test, and no matter which SNPs were removed, there was no effect on the overall MR results, as shown in Figure 1.
Discussion
In the original epidemiological studies, it was shown that increased physical activity was helpful in reducing the risk of some metabolic-related diseases, but some of the studies concluded that there was no association between the two. Such a bias may be due to the confounding factors that were observed in the studies [4]. Although it has been observed in many studies that exercise reduces the risk of hyperlipidaemia, there is controversy over the intensity of exercise. Vento found in his study that directing female university students to increase the amount of time spent exercising at a moderate to high intensity per week reduced plasma levels of total cholesterol and triglycerides to some degree, and because TC and TG are also correlates of the risk of hyperlipidaemia, he concluded that increasing the amount of time spent exercising at a moderate to high intensity [5]. In addition, Chen found in a cross-sectional study of an intermediate to high population that more moderate- to high-intensity exercise was positively associated with a reduction in fasting triglycerides (TG) and total cholesterol (TC), but that increased recreational physical activity such as walking did not affect TG and TC [6]. The conclusions suggested by the above study are consistent with our results in this MR, and the possible hypothesis is that hyperlipidaemia leads to impairment of the PI3K/Akt signalling pathway [7, 8], and that moderate to high intensity exercise up-regulates this signalling pathway, phosphorylating Akt and increasing the phosphorylation level of FoxO1, which serves to inhibit the expression of autophagy-related genes and proteins, such as Beclin1, which is a key component of autophagy. Expression of autophagy-related genes and proteins, such as Beclin1, LC3, and Rab7, and down-regulation of LC3 delayed the autophagosome membrane eye, and down-regulation of Rab7 affected autophagic lysosome maturation in adipose tissue [9]. In addition, up-regulation of the expression of the junctional protein p62 in rat adipose tissue was also observed after the intervention of moderate- to high-intensity exercise in some animal experiments, which also indicated a decrease in the level of lipophagy. Although uncommon in a physician’s clinical diagnosis, it is likely that moderate- to high-intensity exercise improves hyperlipidaemia by promoting FoxO1 phosphorylation to inhibit adipose tissue autophagy, promoting adipocyte metabolism, and decreasing plasma TG and TC levels in hyperlipidaemia patients [10].
In conclusion, our study demonstrated a genetic causal relationship between moderate- to high-intensity exercise and hyperlipidaemia, i.e. increasing moderate- to high-intensity exercise reduces the risk of hyperlipidaemia, which provides evidence, from a genetic point of view, that moderate- to high-intensity exercise improves hyperlipidaemia.