Introduction
Rituximab (RTB) was introduced into rheumatological therapy as a chimeric CD20 immune body of CD20-positive non-Hodgkin’s lymphoma, rheumatoid arthritis (RA) and anti-neutrophil cytoplasmic antibody-associated vasculitis (ANCA-associated vasculitis) [1–4]. In addition, RTB has been used as a second-line treatment for systemic lupus erythematosus (SLE) and has been successfully applied in various autoimmune diseases (AIDs), including as adjuvant therapy after hematopoietic stem cell transplantation [1, 5–7].
Compared to conventional cytotoxic drug therapy, RTB has relatively safe adverse events (AEs) and is well tolerated with long-term use; however, there are some AEs that are becoming known to us, the most common of which are infections, fever, infusion reactions, and hypotension [8–10]. Jens noted that the most common indications for RTB in AIDs were Wegener’s granulomatosis (22.9%), primary Sjogren syndrome (20.0%) and SLE (14.3%), while infection and persistent gammaglobulin abnormalities were the most common AEs, with rates of 34.3% and 25.7%, respectively [11]. Recently, there has been increased awareness of an unusual complication of RTB therapy, late-onset neutropenia (LON) [12–15], which means an absolute blood neutrophil count (ANC) < 1.5 × 109/l occurring at least 4 weeks after the last RTB infusion and extending up to 330 days, and early onset neutropenia (EON) defined as granulocytopenia occurring within 4 weeks [9, 16, 17].
Rituximab-associated neutropenia (RAN), which in most cases is self-resolving in patients and often overlooked by clinicians, is prone to a number of serious complications such as infections that may be life-threatening [13]. Therefore, we should follow up regularly and intervene even in patients with high risk factors such as concomitant old age, shock, clinical evidence of severe infection, severe co-morbidity, allogeneic stem cell transplantation (allo-SCT), or with severe lymphocytopenia or hypogammaglobulinemia [18, 19].
Epidemiology
RAN is uncommon, with the majority of cases being LON rather than EON [8, 12]. This makes EON extremely rare, with only 8 cases reported [9, 17, 20–23]. Specific information on these 8 cases is presented in Table I. In recent years, several retrospective studies have been conducted on LON, and episodes of LON may lead to clinically significant disease and may influence clinical decision making, so it is important that we now have an understanding of the epidemiological and clinical characteristics and management of LON. In several recent reports of hematologic diseases and AIDs, we have listed their epidemiologic and clinical features in Table II based on different case series [19, 24–29]. However, the actual incidence may be higher than noticed due to the asymptomatic course of LON episodes and the possible faster recovery of patients from ANC.
Table I
Information of patients with EON. This table summarizes the early-onset neutropenia of 8 patients after RTB treatment, including patient disease type, personal information, EON onset time and ANC
| Author | Year | Age [years] | Gender | Diagnosis | Treatment plan | Active clinical manifestations | RTB regimen | Onset time of EON after the first RTB [days] | ANC | Treatment of EON |
|---|---|---|---|---|---|---|---|---|---|---|
| Gottenberg [20] | 2005 | 30 | F | SLE | RTB | Pleural pericarditis | 375 mg/m2 ×1 dose | 10 | 0.66 × 109/l | – |
| Gottenberg [20] | 2005 | 22 | F | SLE | RTB + MMF + MP | Joint disease | 375 mg/m2 × 4 dose | 15 | 0.76 × 109/l | – |
| Enriquez [21] | 2007 | 48 | F | SLE | RTB | Multiple arthritis, non-nephrotic proteinuria | 375 mg/m2 × 2 dose | 15 | – | – |
| Arroyo-Avila [9] | 2015 | 36 | F | SLE | RTB + MMF + MP | Oral ulcers, skin rashes, hemolytic anemia and nephrotic syndrome | 375 mg/m2 × 4 dose | 15 | 2.00 × 109/l | – |
| Mealy [22] | 2015 | 32 | F | Neuromyelitis optica | RTB | Extreme fatigue, rectal pain and gum inflammation | 1000 mg | 7 | 0.00 × 109/l | G-CSF (Figstine 300 μg) |
| Mealy [22] | 2015 | 32 | F | Neuromyelitis optica | RTB | Fatigue, fever, oral ulcer | 1000 mg/m2 | 28 | 0.40 × 109/l | G-CSF (Figstine 333 μg) |
| Adler [17] | 2018 | 46 | F | Pemphigus vulgaris | RTB + MMF + Corticosteroid | Refractory oral involvement | 375 mg/m2 × 4 dose | 18 | 0.09 × 109/l | G-CSF, broad-spectrum antibiotics |
| Nelson [23] | 2021 | 65 | M | Mantle cell lymphoma | RTB + Ibrutinib | Neutropenic fever | 375 mg/m2 × 1 dose | 6 | < 0.03 × 103/l | CSF, broad-spectrum antibiotics |
Table II
Onset and clinical characteristics of patients with LON. This table describes personal information and common clinical manifestations of LON in patients with hematological diseases and AIDs after RTB treatment
| Author | Year | Diagnosis | Concomitant treatment | Total patients (N)/age [years]/female (N) | LON cases (N)/IR/age [years]/female (N) | ANC median lowest point | Median time of onset of LON [days] | LON recovery median time [days] | Symptom (N) |
|---|---|---|---|---|---|---|---|---|---|
| Kabei [24] | 2014 | ABO incompatible renal transplantation | IS | 25/50/10 | 12/48%/47/5 | 0.5 × 109/l (range: 0.0–0.9 × 109/l) | 123 (range: 54–348) | 7 (range: 7–42) | – |
| Abdulkader [25] | 2014 | RA | DMARD | 108/64/78 | 5/5%/55/1 | 0.5 × 109/l (range: 0.0–1.3 × 109/l) | 151 (range: 71–184) | 14 (range: 7–15) | Pneumonia 2 |
| Aguiar-Bujanda [19] | 2015 | Non–Hodgkin’s lymphoma | CT | 183/62/100 | 11/6%/60/6 | 0.55 × 109/l (range: 0.06–0.9 × 109/l) | 75 (range: 30–198) | 100 (range: 21–324) | Pneumonia, sepsis and hemorrhagic fever 1 Bronchitis 1 |
| Knight [26] | 2016 | ANCA associated vasculitis | IS | 59/54/35 | 7/12%/54/4 | < 0.1 × 109/l (range: < 0.07 × 109/l) | 86 (range: 56–168) | – | Urinary tract infection 2 Respiratory tract infection 2 Abdominal pain 1 |
| Ha [27] | 2017 | BCL patients receiving ASCT | CT | 315/–/– | 92/29%/–/– | 0.4 × 109/l (range: 0.0–0.9 × 109/l) | 91 (range: 33–166) | 14 (range: 1–233) | Infection 16 Fever 7 Hospitalization for LON 5 Sepsis 1 |
| Rigal [28] | 2020 | MOGAD | – | 25/–/–/ | 4/16%/36/3 | 0.037 × 109/l (range: 0.0–0.1 × 109/l) | 120 (range: 70–186) | 5 (range: 2–11) | – |
| NMOSD | – | 20/–/– | 2/10%/35/2 | ||||||
| MS | – | 340/–/– | 4/1%/51/4 | ||||||
| Boch [29] | 2020 | PF | IS | 25/67/12 | 2/8%/72/2 | 1.42 × 109/l (–) | 127 (range: 95–290) | – | – |
| PV | IS | 92/57/50 | 3/3%/57/2 |
[i] N – number, LON – late-onset neutropenia, IR – incidence rate, ANC – absolute neutrophil count, IS – immunosuppressant, RA – rheumatoid arthritis, DMARD – disease-modifying antirheumatic drugs, MOGAD – MOG-antibody-associated disease, MS – multiple sclerosis, NMOSD – neuromyelitis optica spectrum disorders, BCL – B-cell lymphoma, CT – chemotherapy, ANCA-associated vasculitis – anti-neutrophil cytoplasmic antibody-associated vasculitis, PF – pemphigus foliaceus, PV – pemphigus vulgaris.
Among non-neurological AIDs, a retrospective study found that the prevalence of RA was 1.3–3%, Wegener’s granulomatosis was 23%, and SLE was 20% [30]. Most LON cases occur at a median time of 38 to 175 days after the last RTB dose [31, 32]. Five cases of LON were seen in 108 patients with RA in Abdulkader [25], with a prevalence of 5.63% and a mean time of LON episodes at 151 days. Knight followed up 59 ANCA-associated vasculitis study groups and found that the prevalence of LON was 11.9%, with a mean onset time of 86 days (56–168 days) [26], which is in contrast to Zonozi who proposed a 25% incidence of LON in lupus nephritis compared to our analysis that? supports the same previous data that patients of RA have a lower incidence of LON episodes than patients of SLE or ANCA-associated vasculitis [31, 33]. Only a few cases of LON were found in MOG-antibody-associated disease (MOGAD), multiple sclerosis (MS), and neuromyelitis optica spectrum disorders (NMOSD) [28]. One of the largest cohort studies conducted by Rigal found that compared to MS, patients of NMOSD or MOGAD had a higher incidence of LON, which supported that there may be potential connections among low levels of IgM and neutropenia [28].
Studies have shown that the incidence of LON in hematologic malignancies and AIDs is comparable [34, 35]. LON is regarded as a recognized AE of RTB therapy in lymphoma population, with an incidence of 3–27% [32]. The incidence of LON was much lower in patients with AIDs treated with RTB, only in the range of 1.3–2.3% [30]. Aguiar-Bujanda’s study suggested a 6% incidence of LON episodes in non-Hodgkin’s lymphoma [19]. Ha et al. identified 92 cases of LON in B-cell lymphoma (BCL) patients who underwent autologous hematopoietic stem cell transplantation (ASCT); the incidence of LON was 29.2%, the mean onset time was 91 days (33–166 days), and the mean remission time was 14 days (1–233 days) [27]. The study group divided the patients into two groups, 2004–2008 and 2009–2014, with the former having an incidence of 17.2% and the latter having an incidence of 39.4%, based on risk factor analysis, considering that the increased incidence could be due to the increased use of RTB [27]. Studies have shown that if RTB is given early after ASCT, LON will last longer and be more serious [35, 36].
According to the information of patients in Table II, we classify the etiology of RAN into autoimmune or inflammatory and lymphoma [19, 24–29]. In Table III, the differences in the onset of LON between these two categories of patients are described in terms of the incidence rate, age of onset, onset time (from the last dose of RTB), duration of LON, and ANC median lowest point. We found that there was no significant difference in ANC median lowest point between the two groups, but there were significant differences in the incidence rate, onset time and duration of LON.
Table III
Differences between autoimmune or inflammatory diseases and lymphoma. This table describes the differences between the onset of LON in patients with autoimmune or inflammatory diseases and lymphoma in terms of incidence rate, age of onset, the time from the last use of RTB to the onset of LON, the duration of LON, and ANC
Considering that some patients are not treated with RTB alone, and a large number of other drugs may have been used before, or RTB combined regimen at the same time, the real incidence rate of patients may be disturbed. Due to the lack of routine follow-up of neutrophil counts in most patients within a few months after RTB treatment, this number may underestimate the true incidence rate. In fact, some prospective and retrospective studies have shown that the incidence rate of LON is much higher. Therefore, we have collected relevant data and listed the relevant studies on the comparison of chemotherapy and RTB combined drug regimen in Table IV [13, 37–39]. Our results support that the incidence rate of LON after RTB is significantly higher than that in the chemotherapy group. Analyzing the reason, one possible reason for LON observed in our patients is the correlation between immune disorder after RTB administration and abnormal process of B-cell reconstruction, which leads to immune cachexia in some patients, and then LON [40]. However, neutropenia caused by some cytotoxic drugs is related to bone marrow suppression, which damages stem cells and leads to limited stem cell reserves, which is different from the immunosuppressive effect of RTB. Neutropenia in some autoimmune diseases, such as RA, may also be due to Felty syndrome or large granular lymphoblastic leukemia, but these conditions will lead to long-term neutropenia, rather than the transient situation we observed [25]. Therefore, we consider that LON after using RTB has a greater relationship with RTB.
Table IV
Study on the comparison of chemotherapy and RTB combined drug regimen. This table compares the patient information of chemotherapy with chemotherapy combined with RTB or other treatments
| Author | Diagnosis | Treatment | Patients, n | LON, n (%) | P-value | Median age of LON patients [years] | Median time to neutropenia [days] | Median duration of neutropenia [days] |
|---|---|---|---|---|---|---|---|---|
| Dunleavy [13] | DLBCL, ARL, MCL | DA-EPOCH | 54 | 0 (0) | 0.041 | 44 | 175 | 14 |
| DA-EPOCH-R | 76 | 6 (7.89) | 49 | |||||
| Nitta [37] | CD20+ BCL | Chemotherapy | 52 | 0 (0) | < 0.001 | 62 | 124 | 28 |
| Chemotherapy + R | 107 | 23 (21.50) | 62 | |||||
| Hirayama [38] | DLBCL, FL | Chemotherapy | 18 | 0 (0) | 0.03 | 55 | – | – |
| Chemotherapy + R | 14 | 6 (42.86) | 51 | |||||
| Cattaneo [39] | CD20+ BCL | R | 9 | 3 (33.33) | 0.233 | – | 70 | 77 |
| Chemotherapy + R | 50 | 12 (24) | – | |||||
| Chemotherapy + R + ASCT | 18 | 8 (44.44) | – |
[i] DA-EPOCH – dose-adjusted etoposide–prednisone–Oncovin (vincristine)–cyclophosphamide–hydroxydaunorubicin, R – rituximab, ASCT – autologous hematopoietic stem cell transplantation, DLBCL – diffuse large B-cell lymphoma, MCL – mantle cell lymphoma, ARL – AIDS-related lymphoma, FL – follicular lymphoma.
Risk factors
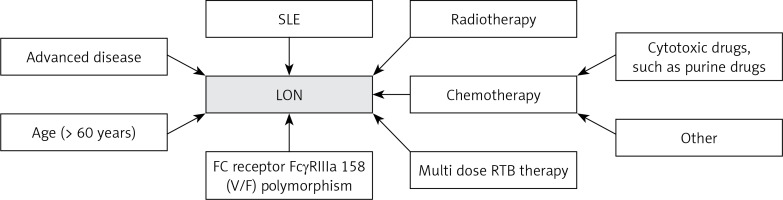
Through a systematic review of the literature, we found that the incidence rate of LON was higher in patients with previous purine analogues or cytotoxic drugs, and those who received more intensive chemotherapy or chemotherapy combined with radiotherapy [32, 37, 38, 41–43]. Specific polymorphisms at position 158 V/F of the IgG Fc receptor FCGR3 have also been reported to be associated, with each additional V allele, the odds of neutropenia tripled [44–48]. In addition, age (> 60 years), advanced disease and having received multiple doses of RTB were also considered risk factors for RAN [18, 32, 39]. Figure 1 summarizes the risk factors for LON.
Figure 1
Risk factors for late-onset neutropenia. This figure summarizes the risk factors for increased susceptibility to LON after RTB treatment
SLE – systemic lupus erythematosus, RTB – rituximab, LON – late-onset neutropenia.
In the largest retrospective cohort of patients with RAN episodes to date, the group found that the highest incidence of RAN was in the first year, with a cumulative incidence of 6.6% in the first year, and that one-fifth of RAN patients had a second episode in the second year after being re-treated with RTB [33]. The risk of LON in patients with SLE is three times higher than in patients with ANCA-associated vasculitis, and the risk of LON is twice as high with low-dose cyclophosphamide combined with RTB induction therapy than without cyclophosphamide [33]. Therefore, it is reasonable to speculate that both SLE and combined cyclophosphamide therapy are independent risk factors for RAN [33].
Mechanisms
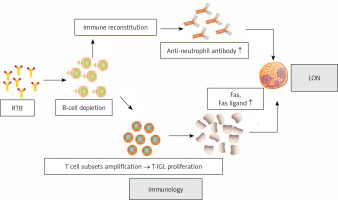
The pathogenesis of RTB-induced EON and LON remains unclear, and many proposed hypotheses attempt to explain the more common phenomenon of LON, which may be caused by a different mechanism than EON [9]. It is unlikely to be a direct toxic effect of RTB. On the one hand, RTB is a chimeric antibody against CD20, while neutrophils and their uncharacterized hematopoietic premise do not express CD20 [48]. On the other hand, because the average half-time of RTB in serum is 31 to 407 h, RAN that occurs after 3 months cannot be explained by its direct toxicity [12, 49, 50]. RTB therapy may affect the balance between granulocytes and lymphocytes in bone marrow. Several hypotheses exist for the mechanism of RAN, including the production of anti-neutrophil antibodies after RTB, disrupted production and delayed neutrophil maturation due to abnormal B-cell reconstitution, and the amplification of T-large granular lymphocyte (T-IGL) populations that may cause granulocyte apoptosis [8, 12, 14, 50–54].
Anti-neutrophil antibodies produced after the use of RTB
Voog et al. proposed the hypothesis that RTB depletes the normal B-lymphocyte population, which stands to recover over a period of 3 to 9 months. During the recovery process, a new immune system is acquired in a non-physiological state. These conditions may be conducive to the production of some transient autoantibodies against neutrophils or their hematopoietic precursors [12]. Figure 2 summarizes the mechanism of antibody-mediated LON by RTB. However, this does not explain the EON.
B-cell reconstitution after RTB leads to the development of neutropenia
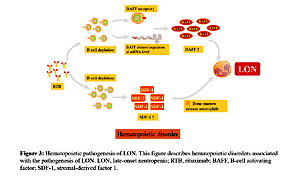
Cytokines produced by stromal cells in the microenvironment play a key role in regulating hematopoietic function [55]. It is speculated that the decrease of neutrophils is regulated by hematopoietic factors [33]. We have found evidence that the loss of B cells will lead to the change of hematopoietic growth factors: the increase of serum B-cell activating factor (BAFF) level during B-cell depletion and the change of stromal-derived factor (SDF-1) concentration can regulate B-cell development and regulate the outflow of neutrophils from bone marrow [56]. Figure 3 describes hematopoietic disorders associated with the pathogenesis of LON [17, 49, 55, 57–59].
Figure 3
Hematopoietic pathogenesis of LON. This figure describes hematopoietic disorders associated with the pathogenesis of LON
LON – late-onset neutropenia, RTB – rituximab, BAFF – B-cell activating factor, SDF-1 – stromal-derived factor 1.
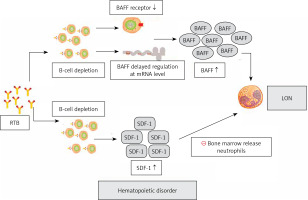
SDF-1 belongs to the chemotactic cytokine family, its chief receptor is CXC chemokine receptor 4 (CXCR4) [60]. The bone marrow is the main place where neutrophils are produced and released to the loop [61]. CXCR4/SDF-1 axis plays a major regulatory role in the development of neutrophils in bone marrow [55, 62]. No matter whether the concentration of SDF-1 in bone marrow is too high or too low, it will affect the release of neutrophils [13]. SDF-1 is also crucial for cell division and migration of early lineage B cells [56, 60]. At 3 months after RTB treatment, we found that the level of circulating SDF-1 was associated with B-cell recovery at 9 months, which may reflect the early increase of SDF-1 in bone marrow during B-cell recovery period [13, 63]. Rapidly expanding B-cell depletion may lead to disruption of the SDF-1 gradient in bone marrow, thereby regulating reduced neutrophil efflux from the bone marrow [61, 64, 65]. Thus, during the recovery of B lymphocytes, the disturbance of SDF-1 delayed the excretion of neutrophils from bone marrow, resulting in LON [13]. We should note that the body of elderly patients may make further efforts to strengthen the excretion of SDF-1 to supplement B cells after RTB therapy compared to young patients [18].
BAFF is a cytokine associated with B-cell recovery, of which neutrophils and monocytes are an important source, and its release is induced by, for example, granulocyte colony-stimulating factor (G-CSF) [66–68]. We hypothesize that two different mechanisms may contribute to increased serum BAFF levels following RTB-induced B-cell depletion: one mechanism is associated with a large reduction in receptors following B-cell depletion [69, 70], and the other is related to delayed regulation of BAFF mRNA levels [71]. BAFF is crucial to stimulate the growth of B cells and is a survival factor for the production of transitional and mature B cells [69]. Terrier et al. hypothesized that competition in the bone marrow that promotes B-cell lymphangiogenesis over granulocyte production caused LON [54]. During LON, when BAFF levels reach a peak during B-cell division, dysplasia of granulocytes in the bone marrow occurs, eventually leading to disrupted granulopoiesis and delayed neutrophil maturation [14, 48, 72].
Expansion of T-LGL populations
The possible role of lymphocyte subpopulation imbalance in the development of LON is noteworthy. Fas ligands are members of the tumor necrosis factor (TNF) family and induce neutrophil apoptosis by binding to their receptor Fas [73]. In lymphoma patients treated with RTB, recent studies have confirmed the data of T cell subsets expansion and imbalance in the presence of B-cell depletion and elimination of B-T cell crosstalk [74–77]. There was significant RTB-induced T-LGL proliferation in RAN patients [74, 75]. While activated as well as tumorigenic T-LGL expresses and secretes large amounts of Fas and Fas ligands, elevated levels of circulating Fas ligands lead to neutropenia [73]. Figure 3 summarizes the mechanism of cell-mediated LON by RTB.
Clinical features and treatment
Most LON cases are self-limiting or even asymptomatic, and a low incidence of serious infectious complications has been reported. Some manifestations of EON are more severe, ranging from sepsis-like manifestations to neutropenia to asymptomatic neutropenia [1, 3]. To date, no deaths associated with EON have been reported [3]. In a retrospective evaluation of non-Hodgkin’s lymphoma by Aguiar-Bujanda et al., 11 patients with LON were identified out of 183 patients [19]. After LON subsided, 4 patients were re-treated with RTB, and 3 of them developed LON relapse [19]. Five patients had LON recurrence during which LON episodes were accompanied by lymphopenia, with 4 patients developing thrombocytopenia and 5 patients developing anemia [19]. Of the 2 LON-related infectious complications [19], 1 patient had acute bronchitis that resolved with oral antibiotics and 1 patient died of pneumonia. Ha et al. identified 2 cases of pneumonia and 14 viral infections in 92 patients with LON who received ASCT. Of these patients, febrile neutropenia was diagnosed in 7 patients, 5 patients were hospitalized, and 1 patient developed sepsis [27]. G-CSF was administered to 37 patients, and no patient died from the infection [27].
Abdulkader et al. described LON in 5 of 108 patients with RA, all 5 of whom had seropositive celiac disease [25]. There was no significant reduction in platelet or red blood cell counts compared to baseline [25]. Two patients developed neutropenia combined with pneumonia, and one of these patients received G-CSF, another patient had a spontaneous recovery of neutrophil count. Two of these patients developed sepsis and required intravenous antibiotics, indicating a significant risk of infection in LON [25]. 11.9% of LON cases were identified in the ANCA-associated vasculitis cohort by Knight, and 2 of the 7 LON cases received TNF prior to RTX. Results showed that 5 patients developed infections and 6 patients were hospitalized [25]. In another cohort, of the 25 patients who successfully received ABO-compatible renal transplants with RTB, Kabei identified 12 patients who developed LON 2–12 months after transplantation, and 5 of these LON cases had biopsy-confirmed acute rejection [24]. Patients with transplantation and acute rejection received a median of four (1-9) G-CSF treatments, and evidence suggests that G-CSF treatment or temporary cessation of mycophenolate mofetil (MMF) treatment is thought to have caused LON to disappear [24].
Currently, for some rare AIDs, Rigal et al. for the first time included RAN for MS as a specific study with 385 patients and found LON episodes in 4 MOGA patients, 2 NMOSD patients, and 4 MS patients [28]. Six of these patients required intravenous antibiotics, 6 patients received concomitant G-CSF therapy, 4 patients were asymptomatic with only neutropenia found in the blood work, and 8 patients had recurrent RAN when they received RTB again after LON remission [28]. Five cases of LON were identified in 117 patients with aspergillosis treated with RTB identified by Boch, patients without specific treatment (antibiotics, granulocyte colony-stimulating factor, or hospitalization) after LON recovery [29].
There is no clear guidance on the need for G-CSF therapy in patients with LON [78]. It is generally accepted that in most cases of grade III RAN, neutrophil recovery is self-limiting and patients recover rapidly without any specific therapy and do not require G-CSF administration [18, 33, 34, 79, 80]. Conversely, some patients may require G-CSF for management, such as infectious complications that are highly likely to occur in patients with grade IV RAN, when G-CSF is justified in patients with grade IV neutropenia and risk factors, usually with rapid response [32, 81]. These risk factors include old age, shock, clinical evidence of severe infection, severe co-morbidity, allogeneic stem cell transplantation (allo-SCT), or the presence of severe lymphopenia or hypogammaglobulinemia [19, 82]. Granulocyte deficiency unresponsive to G-CSF is rare, and how to treat it at this time is controversial [78, 83]. Diez-Feijoo provided a case of Waldenström’s macroglobulinemia unresponsive to G-CSF, where the mechanism of RAN development was considered to be immune-related and effective control was achieved by high-dose intravenous immunoglobulin administration [79]. Because of the risk of re-occurrence of LON, the need for re-treatment with RTB after recovery from LON should be determined on a patient-by-patient basis [19, 32, 35, 39].
By studying the relevant literature, we found that the G-CSF family enhanced the expression of CD20, enhanced the cytotoxicity of neutrophils through antibody primimmune cells. This may improve the antitumor effect of RTB and reduce the severity of chemotherapy-induced bone marrow suppression to improve chemotherapy tolerance [84]. Moreover, the previous clinical research experience of combining RTB and G-CSF also tells us that the response rate of some patients may be increased, and the duration of remission may be prolonged, while the adverse reactions of patients have not increased [85]. At the same time, the increase of neutrophil count may prevent infection. After the onset of LON, the remission time of LON in patients treated with G-CSF is shortened, which is supported by the study of Tesfa et al. [31]. We can boldly guess whether the probability of recurrent LON will also be reduced. We have also studied the literature in related fields, but there is little literature at present, and further research is still needed to support our inference.
Conclusions
RTB is now a part of lymphoma and AID treatment, and LON is one example of a late AE. As the number of patients treated with RTB increases, reports on this component are gradually increasing, and clinicians need to consider this late effect when choosing an appropriate treatment regimen. Most LON cases are reversible and respond well to G-CSF, but can be severe enough to cause life-threatening complications. This article describes the epidemiology, risk factors, pathogenesis, clinical features, and management of this AE, which requires vigilant monitoring because LON may be associated with serious infections.