A 35-year-old female health care worker with IgG deficiency was urgently admitted to our department due to pericardial effusion, which was diagnosed during a follow-up visit in an outpatient clinic. The IgG deficiency was detected over 10 years ago and classified by a hematologist as mild. There was no known history of IgG replacement therapy or chronic or recurrent infections.
Two months prior to admission, she had been urgently hospitalized in a cardio-thoracic center due to an episode of cardiac tamponade evacuated with a pericardiocentesis. She had been discharged after 5 days in good condition and remained asymptomatic until a week before admission to our hospital. The pericardial fluid had been sent to diagnostics, but the test results remained unknown.
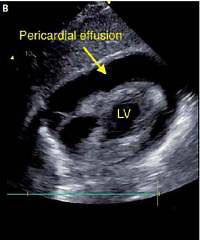
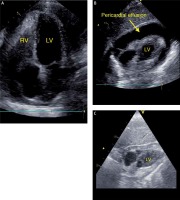
The patient presented fatigue, dyspnea, and heart failure symptoms (New York Heart Association (NYHA) class III). Physical examination revealed tachycardia (heart rate 120 bpm) and hypotension (90/60 mm Hg). The patient denied weight loss, cough, fever, or night chills. Laboratory tests showed anemia (hemoglobin 8.9 mg/dl), elevated C-reactive protein level (CRP 64 mg/dl), no leukocytosis, and elevated CA-125 level (169.3 U/ml, reference < 35 U/ml). In the ECG, sinus rhythm with tachycardia (120 bpm), PR depression in inferior leads (II, III, aVF), and negative T waves in precordial leads (V2-V6) were found. Transthoracic echocardiography (TTE) revealed preserved left ventricular ejection fraction (LVEF, > 60%), pericardial effusion (> 30 mm of fluid), and impaired right ventricular function (right atrium and ventricle were collapsing; Figures 1 A, B). A diagnosis of cardiac tamponade was made, and urgent pericardiocentesis was performed. Over 450 ml of bright yellow, cloudy fluid was evacuated (Figure 1 C). The patient’s condition improved significantly. In the angio-CT, hepatomegaly, a tumor in the uterus (defined by the radiologist as a uterine fibroid), and bilateral pleural effusion were described. Rheumatic diseases and HIV infection were excluded.
Figure 1
A – TTE – 4-chamber view: pericardial effusion, right atrium, and ventricle collapsed. B – TTE – Subcostal 4-chamber view: pericardial effusion. C – TTE – Subcostal 4-chamber view after pericardiocentesis
The pericardial fluid’s microscopic test revealed Mycobacterium tuberculosis, whereas the pleural fluid’s microscopic test for Mycobacterium tuberculosis was negative. Initial anti-tuberculosis treatment was administered, and the patient was transferred to the pulmonology ward for further treatment. The tuberculosis was treated with rifampicin (6 months), pyrazinamide (3 months), ethambutol (3 months), and prednisolone (30 mg daily for 8 weeks) due to pericarditis. Two months after the initiation of TBC treatment, she was urgently admitted to the hospital due to jaundice, which was caused by TBC therapy. The pyrazinamide and rifampicin were discontinued, and the symptoms reversed. The further course of treatment remained complication-free. The follow-up visit was scheduled in order to assess the left ventricular ejection fraction and verify the potential development of constrictive pericarditis. In the follow-up echocardiography 7 months after, there was no fluid in the pericardium, the left ventricular ejection fraction (LVEF) was 60%, and no signs of right ventricular overload or constrictive pericarditis were found.
The latest World Health Organization (WHO) data show that the incidence of tuberculosis in Poland is still higher than in Europe (17 vs. 11 per 100 000 population), but the overall occurrence both in Europe and Poland is declining (4.5% per year). The age profile of patients with tuberculosis has recently changed – the highest incidence rate is observed in middle-aged subjects (45–65 years old), not in the elderly [1].
We present a rare case of isolated tuberculosis pericarditis in a young health care worker with mild IgG deficiency. According to Imazio et al., in developed countries, tuberculosis is responsible for less than 5% of cases of pericarditis [2].
The patient has been working for 8 years as a health care worker and never had any severe infections. Nevertheless, the question of her ability to work in a high infection risk environment and regular contact with ill patients remains controversial. No guidelines address this issue directly. In our opinion, a change of profession or at least workplace to avoid another infection in such patients is necessary.
Our patient also had significantly elevated CA-125, a biomarker usually used for screening for cancer – mainly ovarian. However, high levels of Ca-125 have been reported in patients with pulmonary and extrapulmonary tuberculosis, including pleural, peritoneal, pelvic, miliary, and intra-abdominal disease. It seems that it can be helpful in the diagnosis of both pulmonary and extrapulmonary tuberculosis in challenging clinical situations [3].
In our opinion, every case of pericardial effusion has to be thoroughly examined, and if possible, the cause has to be diagnosed to prevent a recurrence, particularly in patients with immunodeficiency, which has been linked to increased risk of TBC and worse prognosis [4].
Tuberculosis remains a serious problem, including in developed countries. TBC therapy, even though effective, can cause severe complications and is not well tolerated by patients.
According to the ESC Pericarditis Management guidelines and the Investigation of the Management of Pericarditis Trial [5, 6], high dose adjunctive prednisolone reduces the incidence of constrictive pericarditis by 46%. Prednisolone treatment was continued for 8 weeks to avoid the recurrence of effusion/tamponade.
We have discussed steroid treatment in the immunodeficiency patient with a hematology consultant. Steroid treatment is not contraindicated in mild IgG immune deficiency (our patient’s baseline IgG level was 480 mg/dl) and is commonly used in patients with low IgG in other conditions (HIV or leukemia patients).
In conclusion, anti-TBC drugs can rarely cause cardiotoxicity and heart failure; there are a few reports published. None of those adverse events occurred in our case during follow-up [7, 8].