Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL PHARMACOLOGY / RESEARCH PAPER
Acetyl-L-carnitine protects against LPS induced depression via PPAR-γ induced inhibition of NF-κB/NLRP3 pathway
1
Riphah Institute of Pharmaceutical Sciences, Riphah International University, Islamabad, Pakistan
2
College of Natural and Health Sciences, Zayed University, Abu Dhabi, United Arab Emirates
3
Department of Pharmacology, Bannu Medical College, Bannu, KP, Pakistan
4
Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia
5
University of Lahore Islamabad Campus, Islamabad, Pakistan
6
State Key Laboratory of Oncogenomics, School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen, China
Submission date: 2021-08-15
Final revision date: 2021-12-08
Acceptance date: 2021-12-20
Online publication date: 2021-12-27
Corresponding author
Fawad Ali Shah
Riphah Institute of Pharmaceutical Sciences, Riphah International University, Islamabad, Pakistan
Riphah Institute of Pharmaceutical Sciences, Riphah International University, Islamabad, Pakistan
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Major depressive disorder (MDD) is a debilitating human health status characterized by mood swings and high suicidal attempts. Several studies have reported the role of neuroinflammation in MMD, yet the efficacy of natural drug substances on neuroinflammation-associated depression needs to be further investigated. The present study demonstrated the neuroprotective effects of Acetyl-L- carnitine (ALC) alone or in combination with caffeic acid phenethyl ester (CAPE) on lipopolysaccharide (LPS) induced neuro-inflammation, depression, and anxiety-like behavior.
Material and methods:
Male Sprague Dawley (SD) rats were used to explore the relative effects of ALC and the mechanistic interplay of the peroxisome proliferator-activated receptors (PPARγ) in depression. Lipopolysaccharide (LPS) was administered to induce depression and anxiety-like symptoms such as a decreased grooming tendency, diminished locomotive activity, and increased immobility period.
Results:
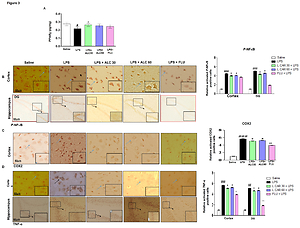
We found marked neuronal alterations in the cortex and hippocampus of LPS intoxicated animals associated with higher inflammatory cytokines expression cyclooxygenase (COX2), tumor necrotic factor-alpha (TNF-α). These detrimental effects exacerbate oxidative stress as documented by a compromised antioxidant system due to high lipid peroxidase (LPO). ALC significantly reverted these changes by positively modulating the PPARγ dependent downstream antioxidant and anti-inflammatory pathways such as NOD and pyrin domain-containing protein 3 (NLRP3) linked nuclear factor kappa B (NF-κB) phosphorylation. Moreover, co-administering NF-κB inhibitor caffeic acid phenethyl ester (CAPE) with ALC also increased PPARγ expression significantly and decreased NF-ᴋB and NLRP3 inflammasome.
Conclusions:
These findings indicate that ALC could be a possible depression supplement. The effects are partly mediated by inhibiting neuroinflammation and NLRP3 inflammasome coupled to PPARγ upregulations.
Major depressive disorder (MDD) is a debilitating human health status characterized by mood swings and high suicidal attempts. Several studies have reported the role of neuroinflammation in MMD, yet the efficacy of natural drug substances on neuroinflammation-associated depression needs to be further investigated. The present study demonstrated the neuroprotective effects of Acetyl-L- carnitine (ALC) alone or in combination with caffeic acid phenethyl ester (CAPE) on lipopolysaccharide (LPS) induced neuro-inflammation, depression, and anxiety-like behavior.
Material and methods:
Male Sprague Dawley (SD) rats were used to explore the relative effects of ALC and the mechanistic interplay of the peroxisome proliferator-activated receptors (PPARγ) in depression. Lipopolysaccharide (LPS) was administered to induce depression and anxiety-like symptoms such as a decreased grooming tendency, diminished locomotive activity, and increased immobility period.
Results:
We found marked neuronal alterations in the cortex and hippocampus of LPS intoxicated animals associated with higher inflammatory cytokines expression cyclooxygenase (COX2), tumor necrotic factor-alpha (TNF-α). These detrimental effects exacerbate oxidative stress as documented by a compromised antioxidant system due to high lipid peroxidase (LPO). ALC significantly reverted these changes by positively modulating the PPARγ dependent downstream antioxidant and anti-inflammatory pathways such as NOD and pyrin domain-containing protein 3 (NLRP3) linked nuclear factor kappa B (NF-κB) phosphorylation. Moreover, co-administering NF-κB inhibitor caffeic acid phenethyl ester (CAPE) with ALC also increased PPARγ expression significantly and decreased NF-ᴋB and NLRP3 inflammasome.
Conclusions:
These findings indicate that ALC could be a possible depression supplement. The effects are partly mediated by inhibiting neuroinflammation and NLRP3 inflammasome coupled to PPARγ upregulations.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.