Introduction
Intracranial aneurysm (IA) is a cerebrovascular lesion characterized by aberrant dilatation of the cerebral artery [1]. The morbidity of IA is approximately 3% of the general population worldwide, about 2% of which will develop into ruptured IA and cause subarachnoid hemorrhage, accounting for approximately 10% of acute cerebral stroke cases [2, 3]. At present, the initiation and progression of IA formation and rupture are complicated. Even more unfortunately, in spite of the advances in techniques of surgical and interventional therapy, the mortality rate and undesirable prognosis in IA patients have failed to improve in clinical practice [3].
Some investigators have attempted to identify the differentially expressed genes between normal superficial temporal arteries (STA) and unruptured or ruptured IA tissues using microarray and polymerase chain reaction (PCR) techniques, suggesting that matrix metalloproteinase-13 (MMP-13) and collagen genes are up-regulated in IA tissues [4]. However, marker in IA tissue as the invasive diagnostic mode limits into routine clinical practice. More recently, there have been a growing number of publications focusing on blood-based non-invasive detection [2, 5], suggesting that numerous microRNAs (miRs) are differentially expressed in peripheral blood of IA patients and can serve as novel biomarkers for the diagnosis of IA.
MiRs as a class of non-coding RNAs perform a fundamental role to regulate gene expression via post-transcriptional repression [6, 7]. Numerous studies have confirmed that miRs participate in a variety of physiological and pathological processes, including cerebrovascular diseases [8, 9]. For example, miR-143, miR-145 and miR-448-3p are implicated in the pathogenesis of IA [10, 11]. Down-regulation of miR-143 and miR-145 in plasma may be associated with IA formation and rupture [12]. Moreover, miRs have proved to be valuable diagnostic markers in various diseases [13, 14]. Some studies also suggest that the underlying mechanism for the alterations in peripheral blood miRs may be associated with the excessive release of miR from the nidus into the circulation [15, 16]. In contrast, all of the cells from the body, not just cells in the nidus, can release miRs into the blood circulation, and nidus-released miRs are not enough to change the miR levels in the blood circulation, because the nidus constitutes only a very small fraction of the body’s cellular mass [17]. Indeed, the changes of miR expression in serum, plasma and whole blood may be a systemic response in the body in response to disease conditions, including deregulated miRs in circulating blood cells [18, 19].
In our study, miRs expression profiling in peripheral whole blood of IA patients was explored using miR microarray assay. Two representative miRs (miR-21 and miR-92) in peripheral whole blood were selected through a comprehensive miRNA array-based approach. Furthermore, we confirmed that up-regulation of miR-21 and miR-92 in peripheral whole blood could serve as non-invasive biomarkers for IA screening.
Material and methods
Patients and specimens
Seventy-three pairs of AI and normal superficial temporal artery (STA) specimens were obtained from 73 IA patients, who underwent microsurgical clipping as described previously [4], from January 2011 to December 2016 at the Guangdong General Hospital (Guangzhou, China). Moreover, peripheral whole blood samples (10 ml) were acquired from 28 healthy controls, seventy-three pre-operative and post-operative IA patients, and stored in an ultra-low temperature refrigerator (Thermo Fisher Scientific). Informed consent forms were collected from all participants. The Ethics Committee of the Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, approved this diagnostic study on January 10th, 2011 (approval number: 201101023).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
miRNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA) was used to extract total RNA, according to the manufacturer’s protocol. TaqMan RT kit and TaqMan MicroRNA assay (Thermo Fisher Scientific, Inc.) were used to perform RT-qPCR assay of miRs, according to the manufacturer’s protocol. miRs expression levels were calculated using the ΔCt method, as previously described [20].
Microarray assays
Microarray analysis was performed using Agilent Human miRNA (8*15K) V14.0 arrays at Ribobio (Guangzhou, China), as described previously [21].
Statistical analysis
Data were presented as the mean ± standard deviation for each group. All statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) as described previously [21]. A p-value less than 0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of healthy controls and IA cases
In our study, 28 healthy controls and seventy-three IA cases, including 34 unruptured and 39 ruptured cases, were recruited for the marker diagnostic study. The systolic blood pressure in both unruptured and ruptured cases was significantly higher than that of the subjects in the healthy control group. Others parameters, including age, gender, diastolic blood pressure, history of hypertension, history of smoking and drinking and fasting blood glucose, had no obvious difference between the two groups. Moreover, we summarized the location of the diagnosed IA as shown in Table I.
Table I
Clinical characteristics of healthy controls and patients with IA
| Paramtere | HC (n = 28) | Unruptured (n = 34) | Ruptured (n = 39) |
|---|---|---|---|
| Age [years] | 51.89 ±5.18 | 52.62 ±5.38 | 51.21 ±5.67 |
| Gender (male/female) | 17/11 | 20/14 | 23/16 |
| Systolic blood pressure [mm Hg] | 116 ±10 | 123 ±15* | 128 ±17** |
| Diastolic blood pressure [mm Hg] | 72 ±7 | 76 ±9 | 80 ±10* |
| History of hypertension | 15 (53.6%) | 18 (52.9%) | 21 (53.8%) |
| History of smoking and drinking | 12 (42.8%) | 20 (58.8%) | 24 (61.5%) |
| Fasting blood glucose [mmol/l] | 5.52 ±1.23 | 5.67 ±1.45 | 5.89 ±1.37 |
| Location: | |||
| Anterior communicating artery | / | 7 | 10 |
| Posterior communicating artery | / | 4 | 3 |
| Anterior cerebral artery | / | 6 | 3 |
| Middle cerebral artery | / | 2 | 4 |
| Posterior cerebral artery | / | 3 | 6 |
| Internal carotid artery | / | 8 | 12 |
| Vertebra artery | / | 4 | 1 |
miR expression profiling in whole blood from healthy controls and IA patients
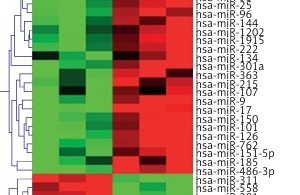
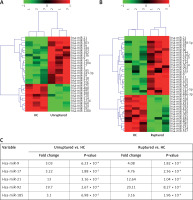
miR microarray assays were performed in nine whole blood samples, which were randomly selected from healthy controls, unruptured or ruptured IA cases (n = 3 in each group). Differentially expressed miRs were filtered based on fold change ≥ 2, p < 0.001 and FDR < 0.001. Compared to healthy controls, 30 miRs were deregulated in whole blood from unruptured IA cases, among which 19 miRs were significantly up-regulated, and 11 miRs were significantly down-regulated (Figure 1 A). In addition, we found that 39 miRs were changed (24 up-regulated and 15 down-regulated) in whole blood from ruptured IA cases compared with the healthy control group (Figure 1 B). Importantly, we found that 5 miRs (miR-9, miR-17, miR-21, miR-92 and miR-185) were simultaneously increased in both unruptured and ruptured IA cases, among which the fold change of miR-21 and miR-92 was obviously higher than miR-9, miR-17 and miR-185 (Figure 1 C). Therefore, we focused on miR-21 and miR-92 in the further experiments.
Figure 1
miR expression profiling in whole blood from healthy controls and IA patients. miR microarray assays were used to measure differentially expressed miRs in whole blood from unruptured (A) or ruptured (B) IA compared with healthy controls (HC) group. Each column represents an individual whole blood sample, and each row represents an individual miR. The color legend at the top of the heat map represents the expression levels of miR, red indicating high expression and green indicating low expression. C – miR microarray assays showed that 5 miRs were up-regulated in both unruptured and ruptured IA compared with HC group
Validation of miR-21 and miR-92 expression by RT-qPCR
To investigate whether miR-21 and miR-92 could function as markers for IA diagnosis, we measured the expression of miR-21 and miR-92 in whole blood from 73 IA cases and 28 healthy controls, as well as in 73 IA tissues and corresponding normal STA. The results demonstrated that miR-21 and miR-92 were significantly higher in IA patients, including whole blood and IA tissues, as compared to the corresponding control group (Figures 2 A, C). However, the expression of miR-21 and miR-92 in whole blood or IA tissues had no statistically significant difference between unruptured and ruptured IA cases (Figures 2 B, D).
Figure 2
Expression of miR-21 and miR-92 was increased in both whole blood and aneurysmal tissues from IA patients. A comparison of miR-21 and miR-92 expression in whole blood was performed between healthy control and IA patients (A), as well as unruptured and ruptured IA (B). A comparison of miR-21 and miR-92 expression was performed between normal STA and IA tissues (C), as well as unruptured and ruptured aneurysmal tissues (D)
*P < 0.05; n.s represents no statistical significance.

Comparison of miR-21 and miR-92 between whole blood and IA tissues
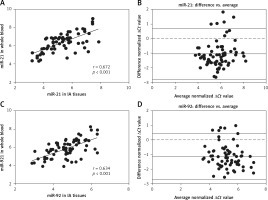
To determine whether miR-21 and miR-92 served as blood-based non-invasive markers for IA, we performed a correlation analysis of miR-21 and miR-92 levels between whole blood and IA tissues. As shown in Figures 3 A and B, the expression of miR-21 between whole blood and IA tissues was significantly and positively correlated in seventy-three IA cases (r = 0.672, p < 0.001), and Bland-Altman analysis showed a high consistency of miR-21 expression in whole blood and IA tissues (mean differences = –1.079 ±0.884; 95% limits of agreement: –2.811, 0.623). We also found that whole blood and IA tissues were strongly correlated for miR-92 expression (r = 0.634, p < 0.001), and Bland-Altman analysis revealed a high concordance of miR-21 expression in whole blood and IA tissues (mean differences = –1.138 ±0.806; 95% limits of agreement: –2.718, 0.442) (Figures 3 C, D). Our results indicated that miR-21 and miR-92 in whole blood accurately reflected the actual yield of miRs in IA tissues, suggesting that both miR-21 and miR-92 were acceptable as blood-based markers for IA screening.
Figure 3
Correlation of miR-21 and miR-92 between whole blood and aneurysmal tissues was investigated. Spearman’s rank analysis and Bland-Altman were used to measure the correlation analysis and consistency check of miR-21 (A, B) and miR-92 (C, D) expression between whole blood and aneurysmal tissues
Evaluation of stabilization of miR-21 and miR-92
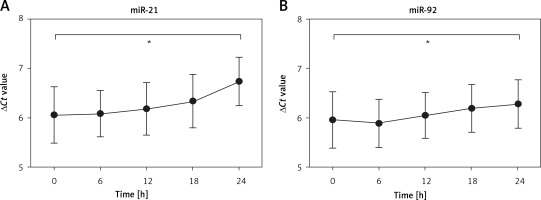
To investigate whether protracted processing of whole blood samples altered the expression of miR-21 and miR-92, we kept the whole blood samples at room temperature for different times. The results demonstrated that the expression of miR-21 and miR-92 had no obvious change at room temperature within 18 h, while the expression levels of miR-21 and miR-92 were significantly lower at 24 h as compared to 0 h (Figures 4 A, B). These results showed a relatively dilatory degradation rate, which provided a high accuracy of analytical results of blood-based miR-21 and miR-92 levels for IA diagnosis.
Comparison of miR-21 and miR-92 in pre-operation and post-operation
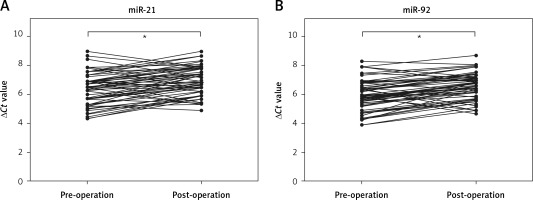
To identify whether miR-21 and miR-92 were released from IA tissues into the circulation, we detected the levels of miR-21 and miR-92 in whole blood before and after the surgical operation. We found that whole blood levels of miR-21 and miR-92 were significantly decreased 14 days after the surgical operation as compared to before the surgical operation in IA patients (Figures 5 A, B). These results suggested that the up-regulation of miR-21 and miR-92 levels in whole blood might be associated with the growth of IA tissues.
Evaluation of miR-21 and miR-92 for IA diagnosis
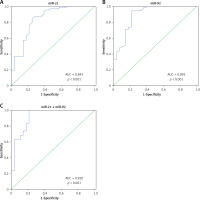
To investigate whether miR-21 and miR-92 could serve as blood-based markers for IA diagnosis, ROC and AUC were performed to evaluate the clinical diagnostic significance of miR-21 and miR-92 in IA patients. The AUC of miR-21 and miR-92 ROC was 0.843 (95% confidence interval (CI): 0.749–0.937) and 0.892 (95% CI: 0.814–0.971) at a diagnostic threshold of 7.33 and 7.51, respectively (Table II, Figures 6 A, B). The sensitivity and specificity of miR-21 and miR-92 for IA diagnosis were 0.849 and 0.750, and 0.945 and 0.786, respectively (Table II, Figures 6 A, B). Furthermore, the ROC analysis was performed in the combination of miR-21 and miR-92. The AUC of the miR-21 combined with miR-92 ROC was 0.920 (95% CI: 0.850–0.988), with sensitivity of 1.000 and specificity of 0.786 at a diagnostic threshold of 18.69 (Table II, Figure 6 C).
Table II
Performance of miR-21 and miR-92 in differential diagnosis of IA from healthy controls
Figure 6
Evaluation of miR-21 and miR-92 for IA diagnosis. Receiver operating characteristics (ROC) curves were drawn with the data of whole blood miR-21 (A) and miR-92 (B) levels from 73 IA patients and 28 healthy controls. Using binary logistic regression analysis, the ROC curves were drawn with the data of whole blood miR-21 combined with miR-92 for IA diagnosis (C)
Discussion
Recently, differentially expressed miRs in whole blood have been detected in both non-malignant conditions, such as type 2 diabetes mellitus [22], rheumatoid arthritis [23] and acute myocardial infarction [24], and cancer conditions, such as liposarcoma [25], lung adenocarcinoma [13] and prostate cancer [26]. Therefore, there have been a growing number of publications focusing on blood-based biomarkers for clinical diagnosis [26]. In the process of IA, numerous miRs are deregulated in aneurysmal tissue and plasma samples and can serve as new biomarkers for IA diagnosis [2, 14, 27]. In the present study, we have renewed interest in whole blood-based miRs for IA diagnosis. IA-related miRs screening was performed in peripheral whole blood based on different expression profiling between healthy controls and IA patients using miR microarray assays. The results demonstrated that up-regulation of miR-21 and miR-92 was identified in whole blood and aneurysmal tissue from IA patients, and then further measurements showed that miR-21 combined with miR-92 exhibited the highest diagnostic power for the diagnosis of IA (AUC = 0.920, sensitivity: 1.000 and specificity: 0.786), indicating that miR-21 combined with miR-92 could be used as combined diagnostic markers for IA.
Previous studies have demonstrated that an increase in levels of immunologic markers, inflammatory cytokines, cell adhesion molecules and enzymes, including complement C3 and C9, interleukin-1β and tumor necrosis factor α (TNF-α), intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM), angiotensin convertase enzyme (ACE) and elastase-to-α1-antitrypsin are seen in aneurysm tissue and blood [1, 28]. It is important to note that these molecules are closely related to brain and vascular damage and aneurysm rupture [1, 29], suggesting that IA development and progression to rupture may be influenced by multiple factors. It is generally believed that complicated pathogenesis of IA leads to a huge challenge of the early detection of IA. Although radiographic imaging of the cerebral vasculature is proved as the only method for aneurysm detection, the asymptomatic clinical presentation and high expense of IA patients reduce the diagnostic rate of IA. Interestingly, biomarkers for noninvasive screening are emerging as effective methods for IA screening [2, 4, 27]. For example, miR-29a is increased in the plasma from IA patients and possesses a high effectiveness in IA diagnosis [27]. A comprehensive analysis of miR expression profiling in plasma from IA patients indicates that 99 miRs are specifically changed in IA patients, suggesting that blood levels of miRs may be useful as a simple and rapid alternative measure for IA screening [2]. In our study, we found that five miRs (miR-9, miR-17, miR-21, miR-92 and miR-185) were up-regulated and participated in IA formation and progression to rupture. Further experiments showed that miR-21 and miR-92 could be used as a diagnostic tool for screening IA patients from apparently healthy individuals. More importantly, a combination of miR-21 and miR-92 for IA screening showed a higher AUC, sensitivity and specificity than miR-21 or miR-92 alone. The expression levels of miR-21 and miR-92 in whole blood were also compared in two subgroups: the unruptured and ruptured aneurysm groups. However, the expression levels of miR-21 and miR-92 showed no significant difference between the two groups. Therefore, whether miR-21 and miR-92 could be indicators for predicting the rupture risk of IA has not been investigated.
Conventional wisdom may suggest that circulating RNAs are unstable because of the high level of RNase activity in plasma [30]. However, Tong et al. revealed that long non-coding RNAs (lncRNAs) are remarkably stable in plasma from cancer patients and can resist RNase A digestion, multiple freeze-thaw cycles and strong acid treatment. The underlining mechanism is that lncRNAs can form relatively stable secondary structures [20]. In the present study, we found that circulating miRs were stable in peripheral whole blood at room temperature within 18 h, which provided plenty of time for clinical examination.
We were surprised to find that miR-21 and miR-92 were significantly lower in whole blood of patients with a surgical operation than before the surgical operation. This result may support the hypothesis that RNAs in blood associate with the microenvironments of pathological tissues [31]. On the other hand, some researchers suggest that blood cells were the major contributor to the up-regulation of RNA in pathologic conditions [32]. Inflammation as a main risk factor accelerates IA development and progression to rupture [33]. A systematic or local inflammatory response can promote miR expression in aneurysmal tissues and whole blood [14, 34]. At present, it is difficult to explain the deregulation of miRs in whole blood, while most researchers consider that blood cells and the metabolism of lesion tissues have come together to regulate miR expression in whole blood [13, 17].
However, there are some limitations in our study: (1) the number of samples was limited, which might lead to the systematic bias of measurement results; (2): the precise mechanism of circulating miRs resistant to endogenous RNase digestion has not been represented in the present study; (3) the secrete mode of whole blood miRs in IA patients did not involve.
In conclusion, the present results demonstrate that miR-21 combined with miR-92 could function as a potential biomarker for IA screening.