Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
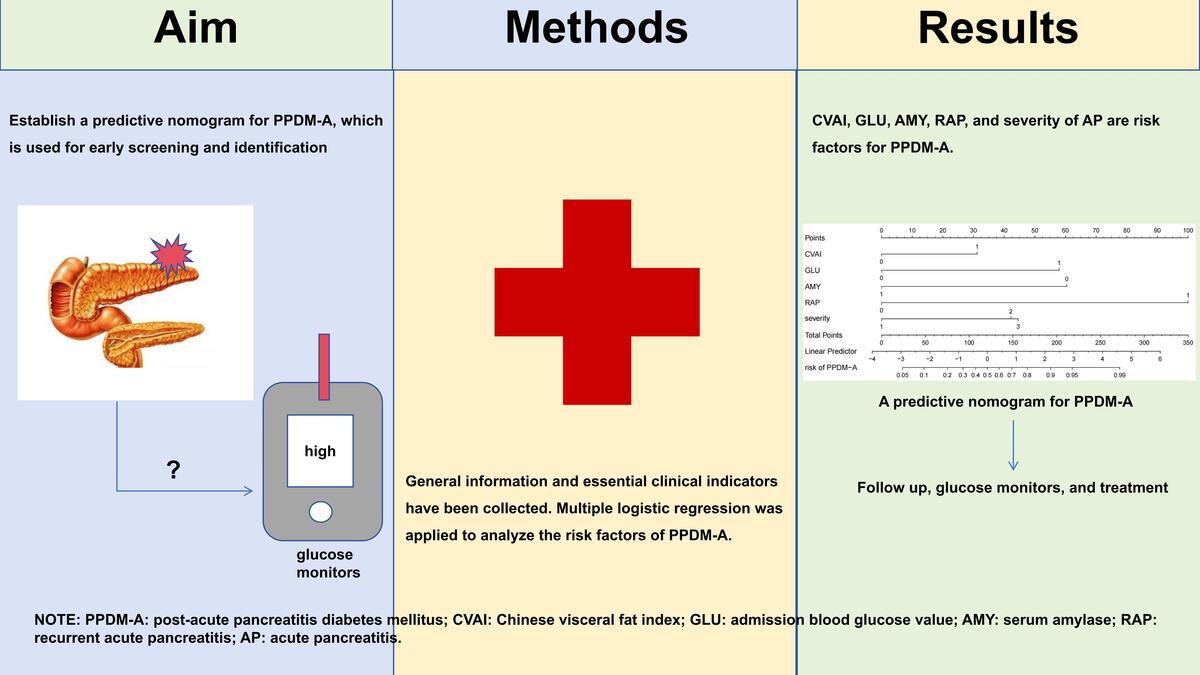
A predictive nomogram for post-acute pancreatitis diabetes mellitus: a retrospective study
1
The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
2
Department of Laboratory Medicine, Southwest Hospital, Third Military Medical University, Chongqing, China
These authors had equal contribution to this work
Submission date: 2023-09-23
Final revision date: 2023-11-14
Acceptance date: 2023-11-26
Online publication date: 2024-08-21
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Post-acute pancreatitis diabetes mellitus (PPDM-A) is a non-negligible sequela of acute pancreatitis (AP), as it has a greater risk of mortality and development of pancreatic cancer compared to type 2 diabetes mellitus (T2DM). Early screening and diagnosis after the onset of pancreatitis are crucial for the outcome of patients. We aimed to establish a predictive nomogram for PPDM-A for early screening and identification.
Material and methods:
A total of 130 patients diagnosed with PPDM-A and 260 gender-matched non-diabetic AP (non-PPDM-A) patients were retrospectively included in this study. They were assigned to a training cohort and a validation cohort with a ratio of 7:3. General information and essential clinical indicators were collected. The Chinese visceral fat index (CVAI) was calculated. Multiple logistic regression was applied to analyze the risk factors of PPDM-A in the training cohort and a predictive model was built. This model was verified in a validation cohort.
Results:
CVAI, admission blood glucose value (GLU), blood amylase (AMY), recurrent acute pancreatitis (RAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis/critical acute pancreatitis (SAP/CAPA) are risk factors for PPDM-A. The area under the curve (AUC) of the prediction model was 0.917. When the cut-off value was 0.356, the sensitivity was 0.888, the specificity was 0.809, and the k was 0.679. The Hosmer-Lemeshow Hosmer test showed a good fit.
Conclusions:
CVAI, GLU, AMY, RAP, and severity of AP are risk factors for PPDM-A. The predictive nomogram established in this study can effectively predict the occurrence of PPDM-A.
Post-acute pancreatitis diabetes mellitus (PPDM-A) is a non-negligible sequela of acute pancreatitis (AP), as it has a greater risk of mortality and development of pancreatic cancer compared to type 2 diabetes mellitus (T2DM). Early screening and diagnosis after the onset of pancreatitis are crucial for the outcome of patients. We aimed to establish a predictive nomogram for PPDM-A for early screening and identification.
Material and methods:
A total of 130 patients diagnosed with PPDM-A and 260 gender-matched non-diabetic AP (non-PPDM-A) patients were retrospectively included in this study. They were assigned to a training cohort and a validation cohort with a ratio of 7:3. General information and essential clinical indicators were collected. The Chinese visceral fat index (CVAI) was calculated. Multiple logistic regression was applied to analyze the risk factors of PPDM-A in the training cohort and a predictive model was built. This model was verified in a validation cohort.
Results:
CVAI, admission blood glucose value (GLU), blood amylase (AMY), recurrent acute pancreatitis (RAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis/critical acute pancreatitis (SAP/CAPA) are risk factors for PPDM-A. The area under the curve (AUC) of the prediction model was 0.917. When the cut-off value was 0.356, the sensitivity was 0.888, the specificity was 0.809, and the k was 0.679. The Hosmer-Lemeshow Hosmer test showed a good fit.
Conclusions:
CVAI, GLU, AMY, RAP, and severity of AP are risk factors for PPDM-A. The predictive nomogram established in this study can effectively predict the occurrence of PPDM-A.
REFERENCES (29)
1.
Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016; 1: 45-55.
2.
Cho J, Petrov MS. Pancreatitis, pancreatic cancer, and their metabolic sequelae: projected burden to 2050. Clin Transl Gastroenterol 2020; 11: e00251.
3.
Jaber S, Garnier M, Asehnoune K, et al. Guidelines for the management of patients with severe acute pancreatitis, 2021. Anaesth Crit Care Pain Med 2022; 41: 101060.
4.
van den Berg FF, Boermeester MA. Update on the management of acute pancreatitis. Curr Opin Crit Care 2023; 29: 145-51.
5.
Yu B, Li J, Li N, et al. Progression to recurrent acute pancreatitis after a first attack of acute pancreatitis in adults. Pancreatology 2020; 20: 1340-6.
6.
Huang W, de la Iglesia-García D, Baston-Rey I, et al. Exocrine pancreatic insufficiency following acute pancreatitis: systematic review and meta-analysis. Dig Dis Sci 2019; 64: 1985-2005.
7.
Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014; 63: 818-31.
8.
ElSayed NA, Aleppo G, Aroda VR, et al. Erratum. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 2023; 46 (Suppl. 1): S19-40. Diabetes Care 2023; 46: 1106.
9.
Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016; 1: 226-37.
10.
Petrov MS. Panorama of mediators in postpancreatitis diabetes mellitus. Curr Opin Gastroenterol 2020; 36: 443-51.
11.
Olesen SS, Svane HML, Nicolaisen SK, et al. Clinical and biochemical characteristics of postpancreatitis diabetes mellitus: a cross-sectional study from the Danish nationwide DD2 cohort. J Diabetes 2021; 13: 960-74.
12.
Cho J, Scragg R, Petrov MS. Postpancreatitis diabetes confers higher risk for pancreatic cancer than type 2 diabetes: results from a Nationwide Cancer Registry. Diabetes Care 2020; 43: 2106-12.
13.
Zhang J, Lv Y, Hou J, et al. Machine learning for post-acute pancreatitis diabetes mellitus prediction and personalized treatment recommendations. Sci Rep 2023; 13: 4857.
14.
Sternby H, Bolado F, Canaval-Zuleta HJ, et al. Determinants of severity in acute pancreatitis: a nation-wide multicenter prospective cohort study. Ann Surg 2019; 270: 348-55.
15.
Xia MF, Chen Y, Lin HD, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep 2016; 6: 38214.
16.
Petrov MS, Taylor R. Intra-pancreatic fat deposition: bringing hidden fat to the fore. Nat Rev Gastroenterol Hepatol 2022; 19: 153-68.
17.
Ren Y, Cheng L, Qie R, et al. Dose-response association of Chinese visceral adiposity index with comorbidity of hypertension and diabetes mellitus among elderly people. Front Endocrinol (Lausanne) 2023; 14: 1187381.
18.
Feng X, Wang J, Wang S, et al. Correlation analysis of anthropometric indices and type 2 diabetes mellitus in residents aged 60 years and older. Front Public Health 2023; 11: 1122509.
19.
Tang M, Wei XH, Cao H, et al. Association between Chinese visceral adiposity index and metabolic-associated fatty liver disease in Chinese adults with type 2 diabetes mellitus. Front Endocrinol (Lausanne) 2022; 13: 935980.
20.
Pan L, Gao Y, Han J, et al. Comparison of longitudinal changes in four surrogate insulin resistance indexes for incident T2DM in middle-aged and elderly Chinese. Front Public Health 2022; 10: 1046223.
21.
Han M, Qin P, Li Q, et al. Chinese visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev 2021; 37: e3370.
22.
Olesen SS, Krarup H, Poulsen JL, et al. Pancreas-specific plasma amylase for assessment and diagnosis of chronic pancreatitis: new insights on an old topic. United European Gastroenterol J 2019; 7: 955-64.
23.
Tu J, Yang Y, Zhang J, et al. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine (Baltimore) 2018; 97: e10713.
24.
Xiao B, Xu HB, Jiang ZQ, Hu JX, Yang GD. Acute pancreatitis in patients with a medical history of type 2 diabetes mellitus: clinical findings and magnetic resonance imaging characteristics. Pancreas 2020; 49: 591-7.
25.
Vipperla K, Papachristou GI, Slivka A, Whitcomb DC, Yadav D. Risk of new-onset diabetes is determined by severity of acute pancreatitis. Pancreas 2016; 45: e14-5.
26.
Walker A, O’Kelly J, Graham C, Nowell S, Kidd D, Mole DJ. Increased risk of type 3c diabetes mellitus after acute pancreatitis warrants a personalized approach including diabetes screening. BJS Open 2022; 6: zrac148.
27.
Avanesov M, Löser A, Smagarynska A, et al. Clinico-radiological comparison and short-term prognosis of single acute pancreatitis and recurrent acute pancreatitis including pancreatic volumetry. PLoS One 2018; 13: e0206062.
28.
Tu J, Zhang J, Ke L, et al. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol 2017; 17: 114.
29.
Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: a systematic review and meta-analysis. Front Physiol 2019; 10: 637.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.