Introduction
Traditional Chinese medicine is widely prescribed for bone disorders in China, and xianlinggubao (XLGB) capsule is a typical one. In 2002, it was approved by the Chinese State Food and Drug Administration (CFDA) officially as an over-the-counter (OTC) drug for the therapy of osteoarthritis (OA), osteoporosis (OP), aseptic osteonecrosis and bone fracture [1], and was further adjusted to an Rx drug in 2017. Preclinical studies demonstrated that XLGB capsule is safe [2] and can effectively prevent the deterioration of musculoskeletal tissues at the hip in the ovariectomized (OVX) rat model [3]. One early multicenter, randomized, open-label, controlled trial based on 180 women with postmenopausal OP showed that treatment with XLGB is safe and can prevent bone loss and increase bone mineral density (BMD) by 2.1% at the lumbar spine at 6 months [1]. Another recent study based on 534 patients with knee and hand OA (272 received XLGB and 262 formed the control group) reported that XLGB capsule significantly improves the function of the knee and hand and also reduces OA associated pain over a 6-month period, in terms of the numeric pain rating scales (NPRS), the Western Ontario and McMaster Universities Arthritis Index, and the Australian/Canadian Osteoarthritis Hand Index scores [4]. These findings confirmed the clinical efficacy of this drug.
However, OA and OP are two distinct diseases. OA is a type of joint disease and is one of the most common forms of chronic arthritis, which is associated with degenerating joint cartilage and underlying bone, while OP is a bone disease that is characterized by reduced bone mass and the deterioration of bone micro-architecture. Therefore, it is necessary to explore the molecular mechanisms of XLGB capsule in the treatment of osteoarthritis and osteoporosis for better improvement of drug administration.
XLGB capsule is a herbal Fufang that consists of six herbs. Their percentages in weight were as follows: Herba epimedii (Chinese name: Yinyanghuo, 70%), Radix dipsaci (Chinese name: Xuduan, 10%), Radix salvia miltiorrhizae (Chinese name: Danshen, 5%), Rhizoma anemarrahenae (Chinese name: Zhimu, 5%), Fructus Psoraleae (Chinese name: Buguzhi, 5%), and Rehmannia glutinosa (Chinese name: Dihuang, 5%) [1]. Chemical analysis of the natural products in these herbs showed that there are hundreds of compounds with varying structures in this formula [1].
Since different compounds may influence different targets, the complex compounds in the capsule might exert therapeutic effects on diseases via synergistically influencing multiple signaling pathways. Therefore, it is relatively difficult to explore the intricate mechanisms of this drug merely using traditional experimental strategies. Network pharmacology is a distinctive new strategy to systematically explore the set of disease-specific proteins and then to investigate chemical molecules that are capable of targeting that set of proteins [5].
In this study, we used a network pharmacology-based approach to explore the complex interactome among the targets of the active compounds of the XLGB capsule.
Material and methods
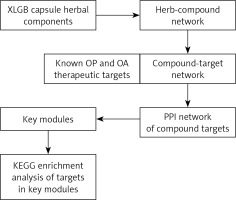
The general workflow for this in-silico analysis of the XLGB capsule in the treatment of OA and OP is shown in Figure 1.
Identification of herbal compounds and the corresponding chemical ingredients in XLGB capsule
To identify the herbal compounds and the corresponding chemical ingredients in XLGB capsule, we performed data mining in BATMAN-TCM, which is an online bioinformatics analysis tool that offers a large amount of information regarding TCM formulae and their chemical ingredients [6]. The key compounds of the five herbs Yinyanghuo, Xuduan, Danshen, Buguzhi and Dihuang were input individually to identify their specific compounds. The compounds without structural information and for which the targets could not be predicted were removed. Then, the targets of each compound were predicted. For screening high potential targets, the score cutoff for a compound was 80. All of the following network and pathway analyses are based on these potential targets.
Identification of the therapeutic targets of osteoarthritis
The therapeutic targets of osteoarthritis were identified using the Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/) [7], which is a database that provides data about the therapeutic targets (both protein and nucleic acid targets) that were approved or studied, their molecular pathway and the corresponding drugs. Osteoarthritis and osteoporosis were used as the key terms to search their therapeutic targets. Only the targets of FDA approved drugs or drugs under clinical studies for OA/OP treatment were identified.
Protein-protein interaction (PPI) analysis
The PPI network was generated using the STRING database (https://string-db.org/, ver. 10.5), which is an online tool for visualizing protein-protein interactions. The minimal interaction score was set to 0.7, which suggests moderate confidence in the interactions.
Module analysis
To gain an insight into the cluster modules in the PPI network, the Cytoscape plugin Molecular Complex Detection (MCODE) [8] was used. The modules were identified using the following parameters: Degree Cutoff: 2, Node Score Cutoff: 0.2, K-Core: 2, Max. Depth: 100. Additionally, the enrichment of the genes in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was analyzed and visualized using the Cytoscape plugin ClueGO. Only the pathways with a p < 0.05 were analyzed.
Results
Herb-compound network
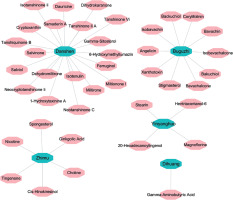
In BATMAN-TCM, we found that the six herbs in XLGB capsule contain 280 compounds, among which 102 compounds do not have structural information and thus their targets cannot be predicted. Therefore, these compounds were removed. Among the compounds subjected to target prediction, 29 were from Buguzhi, 75 were from Danshen, 3 were from Dihuang, 2 were from Xuduan, 37 were from Yinyanghuo, and 32 were from Zhimu. The herb-compound information is provided in Supplementary Table SI.
Among the 178 compounds with known and predicted targets, 41 compounds from 5 herbs had targets with a score over 80. To ensure the high reliability of gene and signaling network construction, only this part of targets was included in the following analysis (Supplementary Table SII). The Herb-Compound network between the five herbs (green) and 41 compounds (pink) is presented in Figure 2.
Compound-target networks
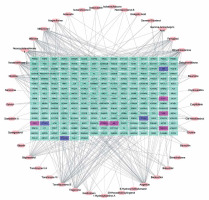
The compound-target network is presented in Figure 2. Bioinformatic prediction showed that there are 246 targets of the 41 compounds. By using osteoarthritis and osteoporosis as the key term to search their therapeutic target, we identified 23 targets of FDA-approved drugs or drugs under clinical studies for OA treatment, including TTD, MMP2, ALOX5, BDKRB2, NR1H4, COX1, COX2, IL1B, PTGS1, PTGS2, SCN1A, TGFB1, TNF, ADAMTS5, BDKRB1, MMP13, IKBKB, IL1A, MAPK12, NOS2, PTGER4, MMP3 and GDF5. We also identified 22 potential therapeutic targets of OP, including AR, CALCR, CNR2, ESR1, CASR, FDPS, GLP2R, PTH, PTH1R, PGR, PTGER2, SRC, TNFSF11, VDR, CTSK, DKK1, IL3RA, LTB4R, PTH2R, SOST, ITGB5 and PTK2B. Intersecting analysis revealed that among the 23 therapeutic targets of OA, 4 were included in the network, including PTGS1, PTGS2, PTGER4 and TNF (blue, Figure 3). PTGS1 and PTGS2 are the targets of miltirone, neotanshinone C, neocryptotanshinone II, tanshiquinone B, dehydromiltirone and miltionone I from Danshen. PTGER4 is a target of 1-hydroxytaxinine A from Danshen. TNF is a target of tanshiquinone B, ferruginol, miltionone I, neocryptotanshinone II and neotanshinone C from Danshen (Supplementary Table SII). Among the 22 therapeutic targets of OP, 6 were included in the network, including AR, ESR1, PGR, PTGER2, TNFSF11 and VDR (purple, Figure 3). AR is a target of iotenulin from Danshen. ESR1 is a target of cis-hinokiresinol and spongesterol from Zhimu; ferruginol, gamma-sitosterol, dihydrokaranone, salvinone and salviol from Danshen; bakuchiol, backuchiol and angelicin from Buguzhi. PGR is a target of angelicin from Buguzhi. PTGER2 is a target of 1-hydroxytaxinine A from Danshen. TNFSF11 is a target of neotanshinone C, miltionone I, neocryptotanshinone II and tanshiquinone B from Danshen. VDR is a target of cryptoxanthin and gamma-sitosterol from Danshen; angelicin and xanthotoxin from Buguzhi; and spongesterol from Zhimu (Supplementary Table SII).
PPI network of compound targets
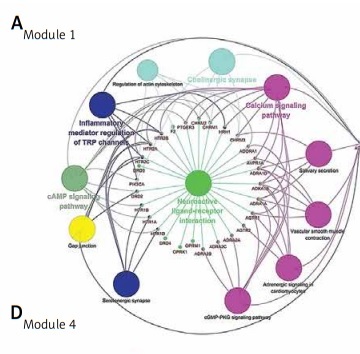
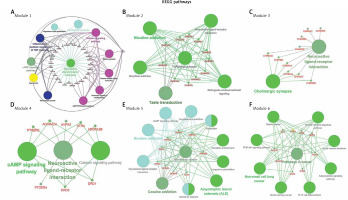
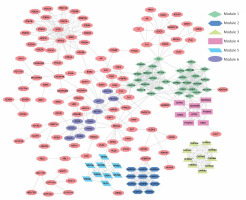
PPI network is a useful tool for visualizing the multiple interactions of diverse proteins. Therefore, the PPI network of the 246 targeting proteins was generated (Figure 4). Their molecular actions were as indicated. In the network, some proteins closely interact and form clustering modules that act together to exert specific molecular functions. Using MCODE [8], we identified six key modules (Figure 5). By performing KEGG analysis, we found that the targets in module 1 were enriched in cAMP signaling pathway, neuroactive ligand-receptor interaction, regulation of actin cytoskeleton, cholinergic synapse, serotonergic synapse, gap junction, inflammatory mediator regulation of TRP channels, calcium signaling pathway, cGMP-PKG signaling pathway, vascular smooth muscle contraction, salivary secretion and adrenergic signaling in cardiomyocytes (Figure 6 A, Supplementary Table SIII). The targets in module 2 were enriched in taste transduction, neuroactive ligand-receptor interaction, GABAergic synapse, retrograde endocannabinoid signaling, morphine addiction and nicotine addiction (Figure 6 B, Supplementary Table SIV). The targets in module 3 were enriched in neuroactive ligand-receptor interaction and cholinergic synapse (Figure 6 C, Supplementary Table SV). The targets in module 4 were enriched in neuroactive ligand-receptor interaction, cAMP signaling pathway and calcium signaling pathway (Figure 6 D, Supplementary Table SVI). The targets in module 5 were enriched in glutamatergic synapse, cocaine addiction, circadian entrainment, long-term potentiation, Alzheimer disease, amyotrophic lateral sclerosis (ALS), alcoholism, cAMP signaling pathway, neuroactive ligand-receptor interaction, amphetamine addiction and nicotine addiction (Figure 6 E, Supplementary Table SVII). The targets in module 6 were enriched in pathways in cancer, PI3K-Akt signaling pathway, Th17 cell differentiation, thyroid hormone signaling pathway, adipocytokine signaling pathway, acute myeloid leukemia, small cell lung cancer, non-small cell lung cancer and gastric cancer (Figure 6 F, Supplementary Table SVIII).
Figure 4
PPI Network of the compound targets. The PPI network of the 246 targeting proteins was generated using string 10.5, by setting the minimal interaction score to 0.7. Their molecular action types are indicated at the bottom of the figure
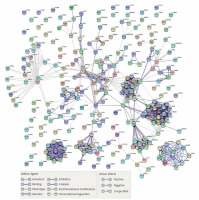
Figure 5
Module analysis of the PPI network. The six key modules in the PPI network were identified using the MCODE app in Cytoscape. Module 1: MCODE score = 15.034; Module 2: MCODE score = 10.8; Module 3: MCODE score = 10.0; Module 4: MCODE score = 8.0; Module 5: MCODE score = 8.0; Module 6: MCODE score = 5.11
Discussion
As a typical traditional Chinese medicine, XLGB has been demonstrated to be an effective and safe treatment for patients with OA or OP [1, 4, 9] and has been approved as an Rx drug by CFDA. However, it is a herb-based drug with complex compounds. Thus, it is meaningful to make further modifications via decreasing the use of herb materials but with equal or even better therapeutic efficacy. This is the key rationale of the current study to explore its molecular mechanisms for a better understanding of its biological effects.
Via network pharmacology-based exploration, we analyzed the components of XLGB capsule and identified 41 components with 246 known and predicted targets. The 246 targets included four known OA targets (PTGS1, PTGS2, PTGER4 and TNF) and six known OP targets (AR, ESR1, PGR, PTGER2, TNFSF11 and VDR). PTGS1 and PTGS2 are targets of FDA-approved OA drugs, such as naproxen [10] and salsalate [11]. PTGER4 antagonist, anti-TNF-α antibody fragment (DLX-105, NCT01624376) and TNF inhibitor (PMI-005, NCT01010919) have been under phase I or II trials. Regarding OP targets, AR is targeted by nandrolone [12] and testosterone [13]. ESR1 is the target of bazedoxifene [14], lasofoxifene [15], Estratab [16] and raloxifene [17]. TNFSF11 is the target of denosumab [18]. One recent study found that ablate estrogen receptor α (ERα) in the medial basal hypothalamus in female mice results in significantly increased BMD of trabecular and cortical bones, suggesting that ESR1 is a critical homeostatic regulator diverting calcium and energy stores [19]. Some other drugs targeting PTGER and VDR are also under clinical trials. These findings indicate that some compounds of XLGB capsule might exert anti-OA and anti-OP effects partly via these targets.
One previous study found that the active components extracted from XLGB such as terpenes from Radix Dipsaci, phenylpropanoids from Herba Epimedii, and coumarins from Fructus Psoraleae can effectively promote the proliferation and mineralization of osteoblast-like UMR 106 cells [20]. However, our in-silico analysis found far more chemical components, which might form quite complex regulatory networks. Different compounds might exert biochemical effects synergistically, which makes the multiple-component capsule more effective than a single reagent in osteogenesis [21]. This mechanism might also apply to the anti-OA effect of the capsule. To further characterize the potential signaling network of XLGB capsule, we identified six key modules in the PPI network. Among them, neuroactive ligand-receptor interaction is a crucial KEGG pathway in modules 1, 2, 3 and 4. The cAMP signaling pathway was identified in modules 1, 4 and 5. The calcium signaling pathway was detected in module 1 and module 4. Therefore, they might be the critical pathways upon which the XLGB capsule might act.
Although several O-associated risk factors such as genetic predisposition, aging, obesity, and joint malalignment have been identified, the pathogenesis of OA remains largely unknown [22]. One recent study compared the dysregulated genes in normal and OA human knee cartilage tissues and found that neuroactive ligand-receptor interaction is among the top pathways in which the dysregulated genes enriched in [22]. Genetic alterations in TGF-β/Smad, Wnt/β-catenin and Indian Hedgehog (Ihh) signaling pathways can result in unbalanced anabolic and catabolic activities in articular cartilage and subsequent irreversible degradation of the extracellular matrix [23]. The cAMP pathway can synergistically modulate TGF-β/Smad-induced expression of extracellular matrix component, thereby regulating extracellular matrix homeostasis [24]. Intracellular calcium can activate the calcium-/calmodulin-dependent protein kinase II (caMK2) and induce the cartilage degeneration of rat mandibular condyle [25] and OA-associated changes in phenotype markers in human chondrocytes [26]. Therefore, we hypothesized that besides the known targets of OA, the active compounds in XLGB capsule might also act upon some targets in these pathways to alleviate OA.
The fundamental cause of OP is the disequilibrium between bone formation and resorption, which are mediated by osteoblasts and osteoclasts, respectively. Therefore, osteoblast-osteoclast communication is critical in maintaining the equilibrium. There are at least three levels of communication: 1) paracrine contact, which includes the secreted chemokines, cytokines, growth factors and other small molecules; 2) direct cellular contact via receptor-ligand interactions on the cell membrane; 3) cell-bone matrix interaction [27]. The paracrine factors include osteoprotegerin, semaphorins (Semas), monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), monocyte chemo-attractants MRP-1 (CCL6), MCP-2 (CCL8) and MCP-5 (CCL12) [27, 28]. Direct osteoblast-osteoclast communication can be mediated via RANKL, Steel factor, ephrin-A2, ephrin-B2, semaphorin 4D, semaphorin 6C, semaphorin 7A and connexins [28, 29]. The bone matrix also acts as a reservoir for a large number of osteotropic factors, such as FGF, BMPs, IGFs, PDGF, TGF-β and VEGF [27, 30]. The transductions of these signaling pathways all involve in neuroactive ligand-receptor interaction. The cAMP signaling pathway has diverse effects on OP, by regulating osteoclast differentiation and function. This pathway can induce the degradation of Cbfa1, which is a transcription factor that acts as a master regulator of osteoblastic differentiation [31]. Also, it induces the expression of RANKL, which enhances osteoclastic differentiation of marrow preosteoclasts [32]. However, some other studies found that in osteoblasts, the cAMP signaling exerts osteogenic effects via regulating multiple genes such as activating transcription factor 4 (Atf4) and aryl hydrocarbon receptor (AhR) [33, 34]. This signaling pathway also contributes to osteoblastic mineralization and subsequent bone formation via glucagon-like peptide-1 (GLP-1) [35]. In addition, activation of cAMP signaling can induce increased osteogenic differentiation of mesenchymal stem cells, at the expense of adipogenesis [36]. These findings collectively suggest that cAMP signaling activity has different effects on osteoclasts, osteoblasts and osteogenesis of mesenchymal stem cells and thus plays a vital role in maintaining the hemostasis between osteoclasts and osteoblasts. Previous studies also showed that in osteoblasts, an increase of intracellular calcium concentration activates the protein kinase Akt, which subsequently enhances osteoblast proliferation and survival [37, 38].
In this study, although we infer that these three KEGG pathways might be the critical pathways upon which XLGB capsule might act, we could not exclude the potential involvement of other KEGG pathways in the anti-OA and anti-OP effects. For example, the cGMP-PKG signaling pathway is associated with the pathophysiologic effects of NO in OA [39]. Some members of the adipocytokine signaling pathways, such as leptin, resistin, and visfatin, are active players in the pathogenesis of OA [40, 41]. The cGMP-PKG signaling pathway, PI3K-Akt signaling pathways, and adipocytokine signaling pathways are also involved in bone formation and resorption [35, 42, 43]. However, some KEGG pathways, such as taste transduction, Alzheimer disease, amyotrophic lateral sclerosis (ALS), acute myeloid leukemia, small cell lung cancer, non-small cell lung cancer and gastric cancer, might be irrelevant to OA and OP pathology. In the future, modification of the capsule might consider a start from relevant components in these pathways. Also, further studies are required to investigate the molecular interactions and functional complementation among the compounds of the herbal materials.
In conclusion, this study provided a systematic regulatory network to show the potential pharmacological mechanisms of XLGB capsule in OA and OP. Neuroactive ligand-receptor interaction, the cAMP signaling pathway, and the calcium signaling pathway might be the critical pathways upon which the capsule might act. These findings also laid down a foundation for future improvement of the drug with the concept “less herbal materials for achieving equal treatment efficacy”. However, more detailed molecular studies should be carried out to validate these findings.